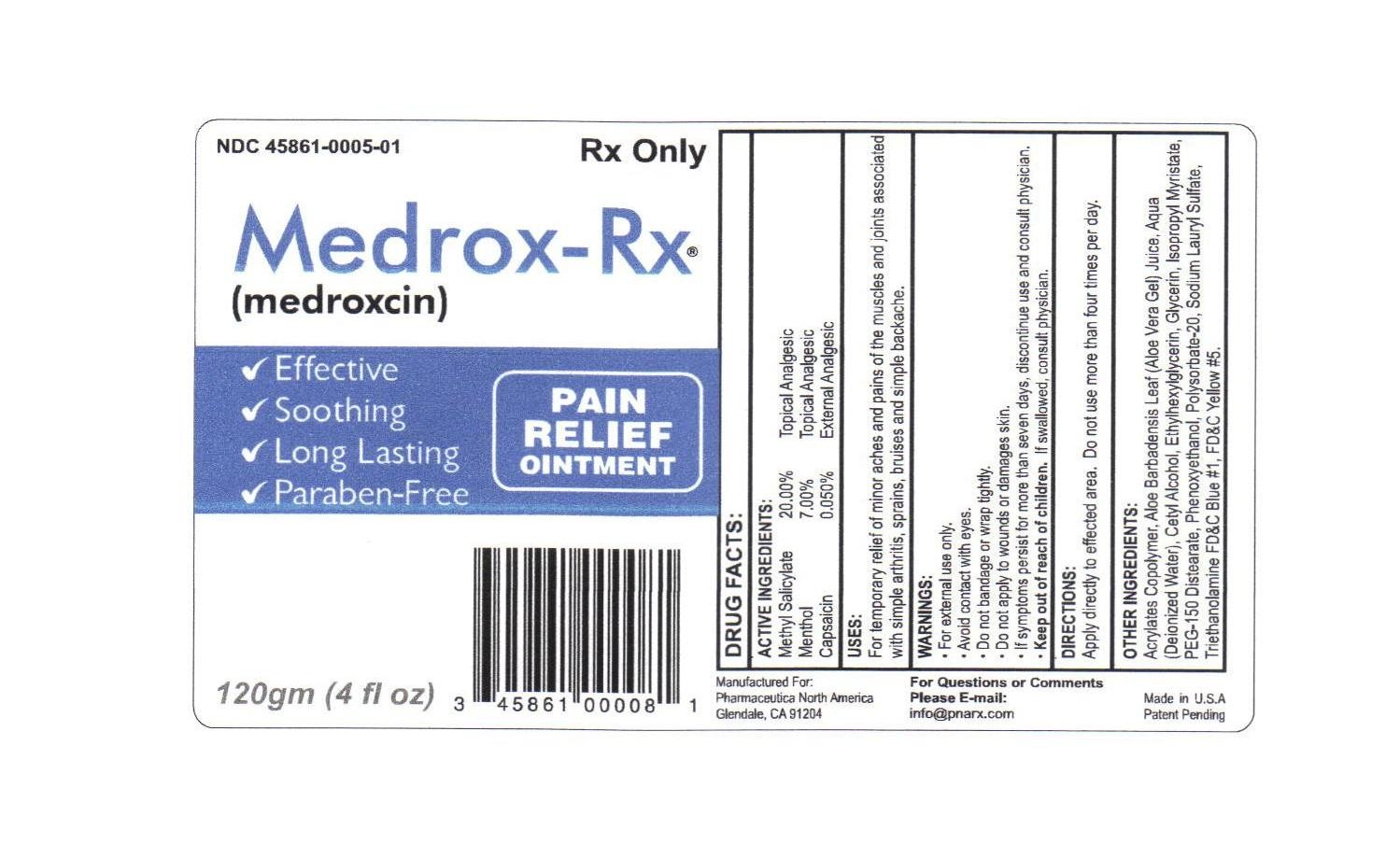
Medrox-Rx
Pharmaceutica North America, Inc.
Pharmaceutica North America, Inc.
Medrox-Rx
FULL PRESCRIBING INFORMATION
Active ingredient
methyl salicylate 20.00 % topical analgesic
menthol 7.00 % topical analgesic
capsaicin 0.050 % external analgesic
acrylates copolymer, aloe barbadensis leaf (aloe vera gel) juice, aqua (deionized water), cetyl alcohol, ethylhexylglycerin, glycerin, isopropyl myristate, peg-150 disterate, phenoxyethanol, polysorbate-20, sodium lauryl sulfate, triethanolamine
keep out of reach of children. if swallowed, consult physician.
apply directly to affected area. do not use more than four times per day.
Purpose
for temporary relief of minor aches and pains of the muscles and joints associated with simple arthritis, sprains, bruises and simple backache.
Uses
for external use only
avoid contact with eyes
do not bandage or wrap tightly
do not apply to wounds or damages skin
if symptoms persist for more than seven days, discontinue use and consult physician
keep out of reach of children. if swallowed consult physician.
Rx Only
Medrox-Rx
(medroxcin) Pain relief ointment
effecive, soothing, long lasting, paraben-free
Medrox-Rxmethyl salicylate, menthol, capsaicin OINTMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||