MAMONDE BRIGHTENING PACT 10HR N21
Mamonde BRIGHTENING POWDER PACT 10HR
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- WARNING
- INGREDIENTS
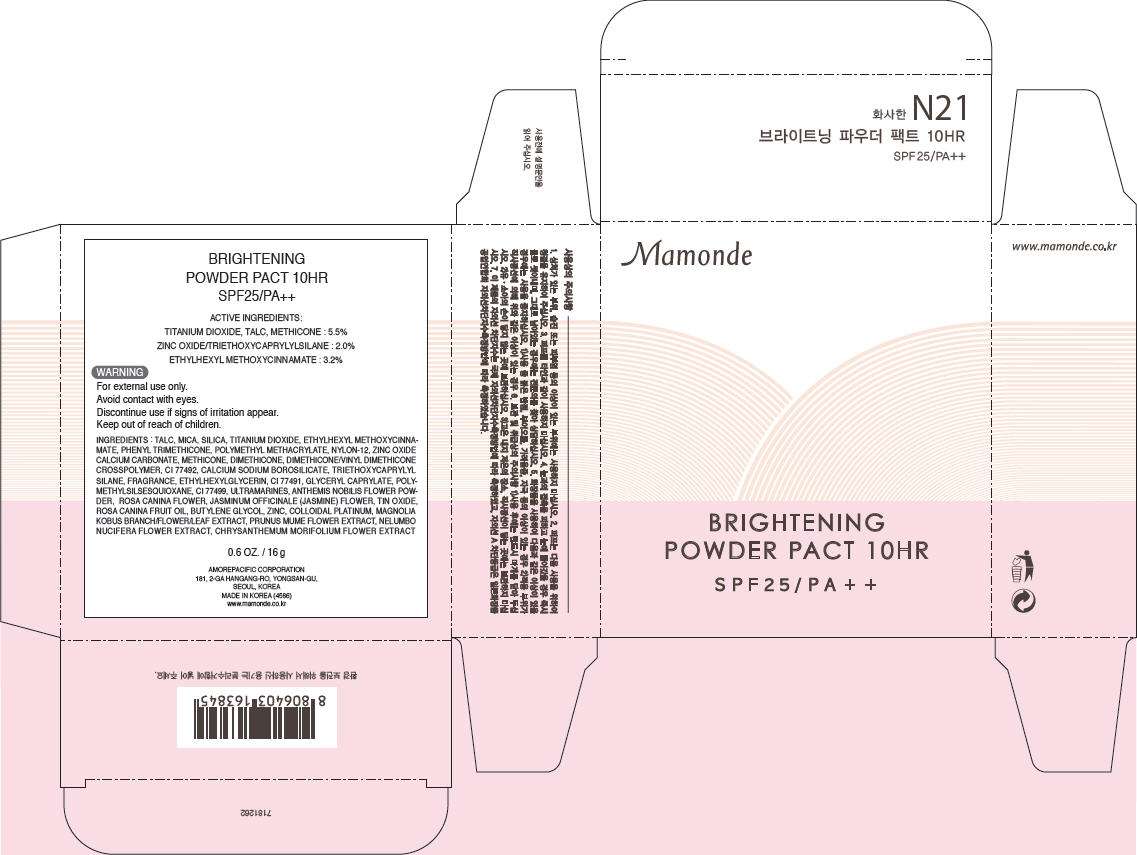
- PRINCIPAL DISPLAY PANEL - 16 g Carton
- PRINCIPAL DISPLAY PANEL - 16 g Carton
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS
TITANIUM DIOXIDE, TALC, METHICONE : 5.5%
ZINC OXIDE/TRIETHOXYCAPRYLYLSILANE : 2.0%
ETHYLHEXYL METHOXYCINNAMATE : 3.2%
WARNING
For external use only.
Avoid contact with eyes.
Discontinue use if signs of irritation appear.
Keep out of reach of children.
INGREDIENTS
TALC, MICA, SILICA, TITANIUM DIOXIDE, ETHYLHEXYL METHOXYCINNAMATE, PHENYL TRIMETHICONE, POLYMETHYL METHACRYLATE, NYLON-12, ZINC OXIDE CALCIUM CARBONATE, METHICONE, DIMETHICONE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, CI 77492, CALCIUM SODIUM BOROSILICATE, TRIETHOXYCAPRYLYL SILANE, FRAGRANCE, ETHYLHEXYLGLYCERIN, CI 77491, GLYCERYL CAPRYLATE, POLYMETHYLSILSESQUIOXANE, CI 77499, ULTRAMARINES, ANTHEMIS NOBILIS FLOWER POWDER, ROSA CANINA FLOWER, JASMINUM OFFICINALE (JASMINE) FLOWER, TIN OXIDE, ROSA CANINA FRUIT OIL, BUTYLENE GLYCOL, ZINC, COLLOIDAL PLATINUM, MAGNOLIA KOBUS BRANCH/FLOWER/LEAF EXTRACT, PRUNUS MUME FLOWER EXTRACT, NELUMBO NUCIFERA FLOWER EXTRACT, CHRYSANTHEMUM MORIFOLIUM FLOWER EXTRACT
0.6 OZ. / 16 g
AMOREPACIFIC CORPORATION
181, 2-GA HANGANG-RO, YONGSAN-GU,
SEOUL, KOREA
MADE IN KOREA (4586)
www.mamonde.co.kr
PRINCIPAL DISPLAY PANEL - 16 g Carton
Mamonde
BRIGHTENING
POWDER PACT 10HR
SPF 25 / PA + +
PRINCIPAL DISPLAY PANEL - 16 g Carton
Mamonde
BRIGHTENING
POWDER PACT 10HR
SPF 25 / PA + +

MAMONDE BRIGHTENING PACT 10HR N21OCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
MAMONDE BRIGHTENING PACT 10HR N23OCTINOXATE, TITANIUM DIOXIDE, and ZINC OXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||