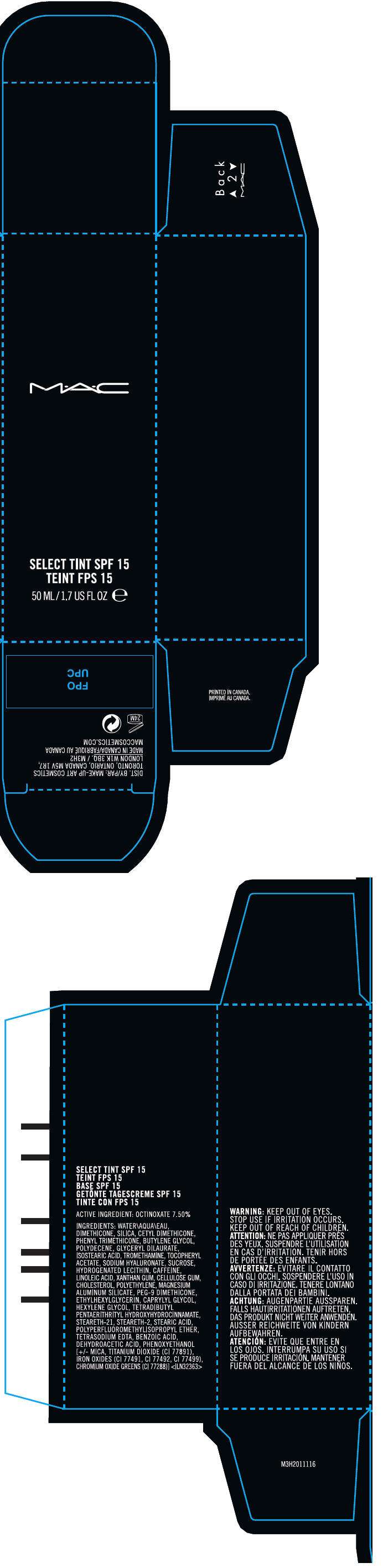
MAC
M•A•C SELECT TINT SPF 15
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT
OCTINOXATE 7.50%
INGREDIENTS
WATER\AQUA\EAU, DIMETHICONE, SILICA, CETYL DIMETHICONE, PHENYL TRIMETHICONE, BUTYLENE GLYCOL, POLYDECENE, GLYCERYL DILAURATE, ISOSTEARIC ACID, TROMETHAMINE, TOCOPHERYL ACETATE, SODIUM HYALURONATE, SUCROSE, HYDROGENATED LECITHIN, CAFFEINE, LINOLEIC ACID, XANTHAN GUM, CELLULOSE GUM, CHOLESTEROL, POLYETHYLENE, MAGNESIUM ALUMINUM SILICATE, PEG-9 DIMETHICONE, ETHYLHEXYLGLYCERIN, CAPRYLYL GLYCOL, HEXYLENE GLYCOL, TETRADIBUTYL PENTAERITHRITYL HYDROXYHYDROCINNAMATE, STEARETH-21, STEARETH-2, STEARIC ACID, POLYPERFLUOROMETHYLISOPROPYL ETHER, TETRASODIUM EDTA, BENZOIC ACID, DEHYDROACETIC ACID, PHENOXYETHANOL [+/- MICA, TITANIUM DIOXIDE (CI 77891), IRON OXIDES (CI 77491, CI 77492, CI 77499), CHROMIUM OXIDE GREENS (CI 77288)]
WARNING
KEEP OUT OF EYES.
STOP USE IF IRRITATION OCCURS.
KEEP OUT OF REACH OF CHILDREN.
DIST. BY/PAR: MAKE-UP ART COSMETICS
TORONTO, ONTARIO, CANADA M5V 1R7,
LONDON W1K 3BQ. / M3H2
PRINCIPAL DISPLAY PANEL - 50 ML Bottle Carton
M•A•C
SELECT TINT SPF 15
50 ML/1.7 US FL OZ e
MACOCTINOXATE LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||