Lytensopril
Lytensopril
FULL PRESCRIBING INFORMATION
USE IN PREGNANCY
When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus. When pregnancy is detected, lisinopril should be discontinued as soon as possible. See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
DESCRIPTION
Lisinopril is an oral long-acting angiotensin converting enzyme inhibitor. Lisinopril, a synthetic peptide derivative, is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-Lproline dihydrate. Its empirical formula is C H N O (2H O and its structural formula is:
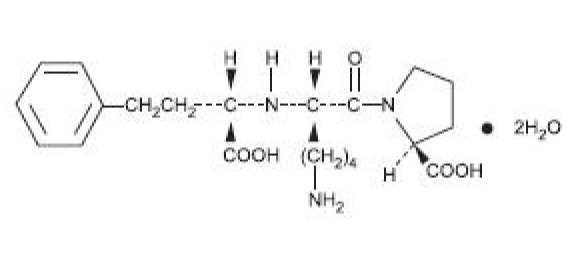
Lisinopril is a white to off-white, crystalline powder, with a molecular weight of 441.53. It is soluble in water and sparingly soluble in methanol and practically insoluble in ethanol. Lisinopril tablets are supplied as 2.5 mg, 5 mg, 10 mg, 20 mg, 30 mg and 40 mg tablets for oral administration.
Inactive Ingredients:
2.5 mg tablets - colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, pre-gelatinized starch, starch. 5 mg, 10 mg, 20 mg and 30 mg tablets – colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, pre-gelatinized starch, red iron oxide, starch. 40 mg tablets - colloidal silicon dioxide, dibasic calcium phosphate, magnesium stearate, mannitol, pre-gelatinized starch, starch, yellow iron oxide.
CLINICAL PHARMACOLOGY
Mechanism of Action
Lisinopril inhibits angiotensin-converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. The beneficial effects of lisinopril in hypertension and heart failure appear to result primarily from suppression of the renin-angiotensin-aldosterone system. Inhibition of ACE results in decreased plasma angiotensin II which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassium. In hypertensive patients with normal renal function treated with lisinopril alone for up to 24 weeks, the mean increase in serum potassium was approximately 0.1 mEq/L; however, approximately 15% of patients had increases greater than 0.5 mEq/L and approximately 6% had a decrease greater than 0.5 mEq/L. In the same study, patients treated with lisinopril and hydrochlorothiazide for up to 24 weeks had a mean decrease in serum potassium of 0.1 mEq/L; approximately 4% of patients had increases greater than 0.5 mEq/L and approximately 12% had a decrease greater than 0.5 mEq/L. (See PRECAUTIONS). Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity.
ACE is identical to kininase, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of lisinopril remains to be elucidated.
While the mechanism through which lisinopril lowers blood pressure is believed to be primarily suppression of the renin-angiotensin-aldosterone system, lisinopril is antihypertensive even in patients with low-renin hypertension. Although lisinopril was antihypertensive in all races studied, Black hypertensive patients (usually a low-renin hypertensive population) had a smaller average response to monotherapy than non-Black patients.
Concomitant administration of lisinopril and hydrochlorothiazide further reduced blood pressure in Black and non-Black patients and any racial differences in blood pressure response were no longer evident.
Pharmacokinetics and Metabolism
Adult Patients
Following oral administration of lisinopril, peak serum concentrations of lisinopril occur within about 7 hours, although there was a trend to a small delay in time taken to reach peak serum concentrations in acute myocardial infarction patients. Declining serum concentrations exhibit a prolonged terminal phase which does not contribute to drug accumulation. This terminal phase probably represents saturable binding to ACE and is not proportional to dose.
Lisinopril does not appear to be bound to other serum proteins. Lisinopril does not undergo metabolism and is excreted unchanged entirely in the urine. Based on urinary recovery, the mean extent of absorption of lisinopril is approximately 25%, with large intersubject variability (6%-60%) at all doses tested (5-80 mg). Lisinopril absorption is not influenced by the presence of food in the gastrointestinal tract. The absolute bioavailability of lisinopril is reduced to 16% in patients with stable NYHA Class II-IV congestive heart failure, and the volume of distribution appears to be slightly smaller than that in normal subjects. The oral bioavailability of lisinopril in patients with acute myocardial infarction is similar to that in healthy volunteers.
Upon multiple dosing, lisinopril exhibits an effective half-life of accumulation of 12 hours.
Impaired renal function decreases elimination of lisinopril, which is excreted principally through the kidneys, but this decrease becomes clinically important only when the glomerular filtration rate is below 30 mL/min. Above this glomerular filtration rate, the elimination half-life is little changed. With greater impairment, however, peak and trough lisinopril levels increase, time to peak concentration increases and time to attain steady state is prolonged. Older patients, on average, have (approximately doubled) higher blood levels and area under the plasma concentration time curve (AUC) than younger patients. (See DOSAGE AND ADMINISTRATION). Lisinopril can be removed by hemodialysis.
Studies in rats indicate that lisinopril crosses the blood-brain barrier poorly. Multiple doses of lisinopril in rats do not result in accumulation in any tissues. Milk of lactating rats contains radioactivity following administration of 14C lisinopril. By whole body autoradiography, radioactivity was found in the placenta following administration of labeled drug to pregnant rats, but none was found in the fetuses.
Pediatric patients
The pharmacokinetics of lisinopril were studied in 29 pediatric hypertensive patients between 6 years and 16 years with glomerular filtration rate > 30 mL/min/1.73 m2. After doses of 0.1 to 0.2 mg/kg, steady state peak plasma concentrations of lisinopril occurred within 6 hours and the extent of absorption based on urinary recovery was about 28%. These values are similar to those obtained previously in adults. The typical value of lisinopril oral clearance (systemic clearance/absolute bioavailability) in a child weighing 30 kg is 10 L/h, which increases in proportion to renal function.
Pharmacodynamics and Clinical Effects
Hypertension
Adult Patients
Administration of lisinopril to patients with hypertension results in a reduction of both supine and standing blood pressure to about the same extent with no compensatory tachycardia. Symptomatic postural hypotension is usually not observed although it can occur and should be anticipated in volume and/or salt-depleted patients. (See WARNINGS). When given together with thiazide-type diuretics, the blood pressure lowering effects of the two drugs are approximately additive.
In most patients studied, onset of antihypertensive activity was seen at one hour after oral administration of an individual dose of lisinopril, with peak reduction of blood pressure achieved by 6 hours. Although an antihypertensive effect was observed 24 hours after dosing with recommended single daily doses, the effect was more consistent and the mean effect was considerably larger in some studies with doses of 20 mg or more than with lower doses; however, at all doses studied, the mean antihypertensive effect was substantially smaller 24 hours after dosing than it was 6 hours after dosing.
In some patients achievement of optimal blood pressure reduction may require two to four weeks of therapy.
The antihypertensive effects of lisinopril are maintained during long-term therapy. Abrupt withdrawal of lisinopril has not been associated with a rapid increase in blood pressure, or a significant increase in blood pressure compared to pretreatment levels.
Two dose-response studies utilizing a once-daily regimen were conducted in 438 mild to moderate hypertensive patients not on a diuretic. Blood pressure was measured 24 hours after dosing. An antihypertensive effect of lisinopril was seen with 5 mg in some patients; however, in both studies blood pressure reduction occurred sooner and was greater in patients treated with 10, 20 or 80 mg of lisinopril. In controlled clinical studies, lisinopril 20-80 mg has been compared in patients with mild to moderate hypertension to hydrochlorothiazide 12.5-50 mg and with atenolol 50-200 mg; and in patients with moderate to severe hypertension to metoprolol 100-200 mg. It was superior to hydrochlorothiazide in effects on systolic and diastolic pressure in a population that was 3/4 Caucasian. Lisinopril was approximately equivalent to atenolol and metoprolol in effects on diastolic blood pressure, and had somewhat greater effects on systolic blood pressure.
Lisinopril had similar effectiveness and adverse effects in younger and older (Greater than 65 years) patients. It was less effective in Blacks than in Caucasians.
In hemodynamic studies in patients with essential hypertension, blood pressure reduction was accompanied by a reduction in peripheral arterial resistance with little or no change in cardiac output and in heart rate. In a study in nine hypertensive patients, following administration of lisinopril, there was an increase in mean renal blood flow that was not significant. Data from several small studies are inconsistent with respect to the effect of lisinopril on glomerular filtration rate in hypertensive patients with normal renal function, but suggest that changes, if any, are not large.
In patients with renovascular hypertension lisinopril has been shown to be well tolerated and effective in controlling blood pressure (See PRECAUTIONS ).
Pediatric Patients
In a clinical study involving 115 hypertensive pediatric patients 6 to 16 years of age, patients who weighed Less than 50 kg received either 0.625, 2.5 or 20 mg of lisinopril daily and patients who weighed ≥ 50 kg received either 1.25, 5, or 40 mg of lisinopril daily. At the end of 2 weeks, lisinopril administered once daily lowered trough blood pressure in a dose-dependent manner with consistent antihypertensive efficacy demonstrated at doses Greater than 1.25 mg (0.02 mg/kg). This effect was confirmed in a withdrawal phase, where the diastolic pressure rose by about 9 mmHg more in patients randomized to placebo than it did in patients who were randomized to remain on the middle and high doses of lisinopril. The dose-dependent antihypertensive effect of lisinopril was consistent across several demographic subgroups: age, Tanner stage, gender, and race. In this study, lisinopril was generally well tolerated.
In the above pediatric studies, lisinopril was given either as tablets or in a suspension for those children and infants who were unable to swallow tablets or who required a lower dose than is available in tablet form.
Heart Failure
During baseline-controlled clinical trials, in patients receiving digitalis and diuretics, single doses of lisinopril resulted in decreases in pulmonary capillary wedge pressure, systemic vascular resistance and blood pressure accompanied by an increase in cardiac output and no change in heart rate.
In two placebo controlled, 12-week clinical studies using doses of lisinopril upto 20 mg, lisinopril as adjunctive therapy to digitalis and diuretics improved the following signs and symptoms due to congestive heart failure: edema, rales, paroxysmal nocturnal dyspnea and jugular venous distention. In one of the studies, beneficial response was also noted for: orthopnea, presence of third heart sound and the number of patients classified as NYHA Class III and IV. Exercise tolerance was also improved in this study. The once-daily dosing for the treatment of congestive heart failure was the only dosage regimen used during clinical trial development and was determined by the measurement of hemodynamic response. A large (over 3000 patients) survival study, the ATLAS Trial, comparing 2.5 and 35 mg of lisinopril in patients with heart failure, showed that the higher dose of lisinopril had outcomes at least as favorable as the lower dose.
Acute Myocardial Infarction
The Gruppo Italiano per lo Studio della Sopravvienza nell’Infarto Miocardico (GISSI-3) study was a multicenter, controlled, randomized, unblinded clinical trial conducted in 19,394 patients with acute myocardial infarction admitted to a coronary care unit. It was designed to examine the effects of short-term (6 week) treatment with lisinopril, nitrates, their combination, or no therapy on short-term (6 week) mortality and on long-term death and markedly impaired cardiac function. Patients presenting within 24 hours of the onset of symptoms who were hemodynamically stable were randomized, in a 2 x 2 factorial design, to six weeks of either 1) lisinopril alone (n=4841), 2) nitrates alone (n=4869), 3) lisinopril plus nitrates (n=4841), or 4) open control (n=4843). All patients received routine therapies, including thrombolytics (72%), aspirin (84%), and a beta-blocker (31%), as appropriate, normally utilized in acute myocardial infarction (MI) patients.
The protocol excluded patients with hypotension (systolic blood pressure ( 100 mmHg), severe heart failure, cardiogenic shock, and renal dysfunction (serum creatinine Greater than2 mg/dL and/or proteinuria Greater than 500 mg/24 h). Doses of lisinopril were adjusted as necessary according to protocol (see DOSAGE AND ADMINISTRATION ).
Study treatment was withdrawn at six weeks except where clinical conditions indicated continuation of treatment.
The primary outcomes of the trial were the overall mortality at 6 weeks and a combined end point at 6 months after the myocardial infarction, consisting of the number of patients who died, had late (day 4) clinical congestive heart failure, or had extensive left ventricular damage defined lisinopril (n=9646), alone or with nitrates, had an 11% lower risk of death (2p [two-tailed] = 0.04) compared to patients receiving no lisinopril (n=9672) (6.4% vs. 7.2%, respectively) at six weeks. Although patients randomized to receive lisinopril for up to six weeks also fared numerically better on the combined end point at 6 months, the open nature of the assessment of heart failure, substantial loss to follow-up echocardiography, and substantial excess use of lisinopril between 6 weeks and 6 months in the group randomized to 6 weeks of lisinopril, preclude any conclusion about this end point.
Patients with acute myocardial infarction, treated with lisinopril, had a higher (9% versus 3.7%) incidence of persistent hypotension (systolic blood pressure Less than 90 mmHg for more than 1 hour) and renal dysfunction (2.4% versus 1.1%) in-hospital and at six weeks (increasing creatinine concentration to over 3 mg/dL or a doubling or more of the baseline serum creatinine concentration). See ADVERSE REACTIONS Acute Myocardial Infarction.
Uses
INDICATIONS AND USAGE
Hypertension
Lisinopril tablets are indicated for the treatment of hypertension. They may be used alone as initial therapy or concomitantly with other classes of antihypertensive agents.
Heart Failure
Lisinopril tablets are indicated as adjunctive therapy in the management of heart failure in patients who are not responding adequately to diuretics and digitalis.
Acute Myocardial Infarction
Lisinopril tablets are indicated for the treatment of hemodynamically stable patients within 24 hours of acute myocardial infarction, to improve survival. Patients should receive, as appropriate, the standard recommended treatments such as thrombolytics, aspirin and beta-blockers.
In using lisinopril tablets, consideration should be given to the fact that another angiotensin-converting enzyme inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen vascular disease, and that available data are insufficient to show that lisinopril tablets does not have a similar risk. (See WARNINGS).
In considering the use of lisinopril tablets, it should be noted that in controlled clinical trials ACE inhibitors have an effect on blood pressure that is less in Black patients than in non-Blacks. In addition, ACE inhibitors have been associated with a higher rate of angioedema in Black than in non-Black patients (see WARNINGS, Anaphylactoid and Possibly Related Reactions ).
CONTRAINDICATIONS
Lisinopril is contraindicated in patients who are hypersensitive to this product and in patients with a history of angioedema related to previous treatment with an angiotensin converting enzyme inhibitor and in patients with hereditary or idiopathic angioedema.
PRECAUTIONS
General
Aortic Stenosis/ Hypertrophic Cardiomyopathy
As with all vasodialators, lisinopril should be given with caution to patients with obstruction in the outflow tract of the left ventricle.
Impaired Renal Function
As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals. In patients with severe congestive heart failure whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with angiotensin converting enzyme inhibitors, including lisinopril, may be associated with oliguria and/or progressive azotemia and rarely with acute renal failure and/or death.
In hypertensive patients with unilateral or bilateral renal artery stenosis, increases in blood urea nitrogen and serum creatinine may occur. Experience with another angiotensin-converting enzyme inhibitor suggests that these increases are usually reversible upon discontinuation of lisinopril and/or diuretic therapy. In such patients, renal function should be monitored during the first few weeks of therapy.
Some patients with hypertension or heart failure with no apparent pre-existing renal vascular disease have developed increases in blood urea nitrogen and serum creatinine, usually minor and transient, especially when lisinopril has been given concomitantly with a diuretic. This is more likely to occur in patients with pre-existing renal impairment. Dosage reduction and/or discontinuation of the diuretic and/or lisinopril may be required.
Patients with acute myocardial infarction in the GISSI-3 trial treated with lisinopril had a higher (2.4% versus 1.1%) incidence of renal dysfunction in-hospital and at six weeks (increasing creatinine concentration to over 3 mg/dL or a doubling or more of the baseline serum creatinine concentration). In acute myocardial infarction, treatment with lisinopril should be initiated with caution in patients with evidence of renal dysfunction, defined as serum creatinine concentration exceeding 2 mg/dL. If renal dysfunction develops during treatment with lisinopril (serum creatinine concentration exceeding 3 mg/dL or a doubling from the pre-treatment value) then the physician should consider withdrawal of lisinopril.
Evaluation of patients with hypertension, heart failure, or myocardial infarction should always include assessment of renal function. (See DOSAGE AND ADMINISTRATION).
Hyperkalemia
In clinical trials hyperkalemia (serum potassium greater than 5.7 mEq/L) occurred in approximately 2.2% of hypertensive patients and 4.8% of patients with heart failure. In most cases these were isolated values which resolved despite continued therapy. Hyperkalemia was a cause of discontinuation of therapy in approximately 0.1% of hypertensive patients; 0.6% of patients with heart failure and 0.1% of patients with myocardial infarction. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements and/or potassium-containing salt substitutes. Hyperkalemia can cause serious, sometimes fatal, arrhythmias. Lisinopril should be used cautiously, if at all, with these agents and with frequent monitoring of serum potassium (See PRECAUTIONS, Drug Interactions).
Cough
Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been reported with all ACE inhibitors, almost always resolving after discontinuation of therapy. ACE inhibitor-induced cough should be considered in the differential diagnosis of cough.
Surgery/Anesthesia
In patients undergoing major surgery or during anesthesia with agents that produce hypotension, lisinopril may block angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion.
Information for Patients
Angioedema
Angioedema, including laryngeal edema, may occur at any time during treatment with angiotensin-converting enzyme inhibitors, including lisinopril. Patients should be so advised and told to report immediately any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physician.
Symptomatic Hypotension
Patients should be cautioned to report lightheadedness especially during the first few days of therapy. If actual syncope occurs, the patient should be told to discontinue the drug until they have consulted with the prescribing physician.
All patients should be cautioned that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of reduction in fluid volume. Other causes of volume depletion such as vomiting or diarrhea may also lead to a fall in blood pressure; patients should be advised to consult with their physician.
Hyperkalemia
Patients should be told not to use salt substitutes containing potassium without consulting their physician.
Hypoglycemia
Diabetic patients treated with oral antidiabetic agents or insulin starting an ACE inhibitor should be told to closely monitor for hypoglycemia, especially during the first month of combined use. (See PRECAUTIONS, Drug Interactions .)
Leukopenia/Neutropenia
Patients should be told to report promptly any indication of infection (e.g., sore throat, fever) which may be a sign of leukopenia/neutropenia.
Pregnancy
Female patients of childbearing age should be told about the consequences of exposure to ACE inhibitors during pregnancy. These patients should be asked to report pregnancies to their physicians as soon as possible.
NOTE: As with many other drugs, certain advice to patients being treated with lisinopril is warranted. This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Drug Interactions
Hypotension - Patients on Diuretic Therapy
Patients on diuretics and especially those in whom diuretic therapy was recently instituted, may occasionally experience an excessive reduction of blood pressure after initiation of therapy with lisinopril. The possibility of hypotensive effects with lisinopril can be minimized by either discontinuing the diuretic or increasing the salt intake prior to initiation of treatment with lisinopril. If it is necessary to continue the diuretic, initiate therapy with lisinopril at a dose of 5 mg daily, and provide close medical supervision after the initial dose until blood pressure has stabilized (See WARNINGS, and DOSAGE AND ADMINISTRATION). When a diuretic is added to the therapy of a patient receiving lisinopril, an additional antihypertensive effect is usually observed. Studies with ACE inhibitors in combination with diuretics indicate that the dose of the ACE inhibitor can be reduced when it is given with a diuretic. (See DOSAGE AND ADMINISTRATION).
Antidiabetics
Epidemiological studies have suggested that concomitant administration of ACE inhibitors and antidiabetic medicines (insulins, oral hypoglycemic agents) may cause an increased blood-glucose-lowering effect with risk of hypoglycemia. This phenomenon appeared to be more likely to occur during the first weeks of combined treatment and in patients with renal impairment. In diabetic patients treated with oral antidiabetic agents or insulin, glycemic control should be closely monitored for hypoglycemia, especially during the first month of treatment with an ACE inhibitor.
Non-steroidal Anti-inflammatory Agents
In some patients with compromised renal function who are being treated with non-steroidal anti-inflammatory drugs, the co-administration of lisinopril may result in further deterioration of renal function. These effects are usually reversible. In a study in 36 patients with mild to moderate hypertension where the antihypertensive effects of lisinopril alone were compared to lisinopril given concomitantly with indomethacin, the use of indomethacin was associated with a reduced effect, although the difference between the two regimens was not significant.
Other Agents
Lisinopril has been used concomitantly with nitrates and/or digoxin without evidence of clinically significant adverse interactions. This included post myocardial infarction patients who were receiving intravenous or transdermal nitroglycerin. No clinically important pharmacokinetic interactions occurred when lisinopril was used concomitantly with propranolol or hydrochlorothiazide. The presence of food in the stomach does not alter the bioavailability of lisinopril.
Agents Increasing Serum Potassium
Lisinopril attenuates potassium loss caused by thiazide-type diuretics. Use of lisinopril with potassium-sparing diuretics (e.g., spironolactone, eplerenone, triamterene or amiloride), potassium supplements, or potassium-containing salt substitutes may lead to significant increases in serum potassium. Therefore, if concomitant use of these agents is indicated because of demonstrated hypokalemia, they should be used with caution and with frequent monitoring of serum potassium. Potassium sparing agents should generally not be used in patients with heart failure who are receiving lisinopril.
Lithium
Lithium toxicity has been reported in patients receiving lithium concomitantly with drugs which cause elimination of sodium, including ACE inhibitors. Lithium toxicity was usually reversible upon discontinuation of lithium and the ACE inhibitor. It is recommended that serum lithium levels be monitored frequently if lisinopril is administered concomitantly with lithium.
Gold
Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy including lisinopril.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of a tumorigenic effect when lisinopril was administered for 105 weeks to male and female rats at doses up to 90 mg/kg/day (about 56 or 9 times* the maximum recommended daily human dose, based on body weight and body surface area, respectively). There was no evidence of carcinogenicity when lisinopril was administered for 92 weeks to (male and female) mice at doses up to 135 mg/kg/day (about 84 times* the maximum recommended daily human dose). This dose was 6.8 times the maximum human dose based on body surface area in mice.
Lisinopril was not mutagenic in the Ames microbial mutagen test with or without metabolic activation. It was also negative in a forward mutation assay using Chinese hamster lung cells. Lisinopril did not produce single strand DNA breaks in an in vitro alkaline elution rat hepatocyte assay. In addition, lisinopril did not produce increases in chromosomal aberrations in an in vitro test in Chinese hamster ovary cells or in an in vivo study in mouse bone marrow.
There were no adverse effects on reproductive performance in male and female rats treated with up to 300 mg/kg/day of lisinopril. This dose is 188 times and 30 times the maximum human dose when based on mg/kg and mg/m2, respectively.
*Calculations assume a human weight of 50 kg and human body surface area of 1.62 m2.
Pregnancy
Pregnancy Categories C (first trimester) and D (second and third trimesters). See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
Nursing Mothers
Milk of lactating rats contains radioactivity following administration of 14C lisinopril. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ACE inhibitors, a decision should be made whether to discontinue nursing or discontinue lisinopril, taking into account the importance of the drug to the mother.
Pediatric Use
Antihypertensive effects of lisinopril have been established in hypertensive pediatric patients aged 6 to 16 years.
There are no data on the effect of lisinopril on blood pressure in pediatric patients under the age 6 or in pediatric patients with glomerular filtration rate less than 30 mL/min/1.73 m2 (See CLINICAL PHARMACOLOGY, Pharmacokinetics and Metabolism and Pharmacodynamics and Clinical Effects, and DOSAGE AND ADMINISTRATION .)
Geriatic Use
Clinical studies of lisinopril in patients with hypertension did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other clinical experience in this population has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
In the ATLAS trial of lisinopril in patients with congestive heart failure, 1,596 (50%) were 65 and over, while 437 (14%) were 75 and over. In a clinical study of lisinopril in patients with myocardial infarctions 4,413 (47%) were 65 and over, while 1,656 (18%) were 75 and over. In these studies, no overall differences in safety or effectiveness were observed between elderly and younger patients, and other reported clinical experiences has not identified differences in responses between the elderly and younger patients (see CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Heart Failure and CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Acute Myocardial Infarction ).
Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Pharmacokinetic studies indicate that maximum blood levels and area under the plasma concentration time curve (AUC) are doubled in older patients (see CLINICAL PHARMACOLOGY, Pharmacokinetics and Metabolism ).
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. Evaluation of patients with hypertension, congestive heart failure, or myocardial infarction should always include assessment of renal function (see DOSAGE AND ADMINISTRATION ).
ADVERSE REACTIONS
Lisinopril has been found to be generally well tolerated in controlled clinical trials involving 1969 patients with hypertension or heart failure. For the most part, adverse experiences were mild and transient.
Hypertension
In clinical trials in patients with hypertension treated with lisinopril, discontinuation of therapy due to clinical adverse experiences occurred in 5.7% of patients. The overall frequency of adverse experiences could not be related to total daily dosage within the recommended therapeutic dosage range.
For adverse experiences occurring in greater than 1% of patients with hypertension treated with lisinopril or lisinopril plus hydrochlorothiazide in controlled clinical trials, and more frequently with lisinopril and/or lisinopril plus hydrochlorothiazide than placebo, comparative incidence data are listed in the table below:
|
|
Lisinopril (n=1349) Incidence (discontinuation) |
Lisinopril/ Hydrochlorothiazide (n=629) Incidence (discontinuation) |
Placebo (n=207) Incidence (discontinuation) |
| Body as a Whole |
|
|
|
| Fatigue | 2.5 (0.3) | 4.0 (0.5) | 1.0 (0.0) |
| Asthenia | 1.3 (0.5) |
2.1 (0.2) |
1.0 (0.0) |
| Orthostatic Effects | 1.2 (0.0) |
3.5 (0.2) | 1.0 (0.0) |
| Cardiovascular |
|
|
|
| Hypotension | 1.2 (0.5) |
1.6 (0.5) | 0.5 (0.5) |
| Digestive |
|
|
|
| Diarrhea | 2.7 (0.2) | 2.7 (0.3) | 2.4 (0.0) |
| Nausea | 2.0 (0.4) | 2.5 (0.2) |
2.4 (0.0) |
| Vomiting | 1.1 (0.2) | 1.4 (0.1) | 0.5 (0.0) |
| Dyspepsia | 0.9 (0.0) |
1.9 (0.0) | 0.0 (0.0) |
| Musculoskeletal |
|
|
|
| Muscle Cramps | 0.5 (0.0) | 2.9 (0.8) | 0.5 (0.0) |
| Nervous/Psychiatric |
|
|
|
| Headache | 5.7 (0.2) | 4.5 (0.5) | 1.9 (0.0) |
| Dizziness | 5.4 (0.4) | 9.2 (1.0) |
1.9 (0.0) |
| Paresthesia | 0.8 (0.1) | 2.1 (0.2) | 0.0 (0.0) |
| Decreased Libido | 0.4 (0.1) | 1.3 (0.1) | 0.0 (0.0) |
| Vertigo | 0.2 (0.1) |
1.1 (0.2) | 0.0 (0.0) |
| Respiratory |
|
|
|
| Cough | 3.5 (0.7) |
4.6 (0.8) |
1.0 (0.0) |
| Upper Respiratory Infection | 2.1 (0.1) | 2.7 (0.1) |
0.0 (0.0) |
| Common Cold | 1.1 (0.1) |
1.3 (0.1) | 0.0 (0.0) |
| Nasal Congestion | 0.4 (0.1) | 1.3 (0.1) | 0.0 (0.0) |
| Influenza | 0.3 (0.1) |
1.1 (0.1) | 0.0 (0.0) |
| Skin |
|
|
|
| Rash | 1.3 (0.4) | 1.6 (0.2) | 0.5 (0.5) |
| Urogenital |
|
|
|
| Impotence | 1.0 (0.4) |
1.6 (0.5) | 0.0 (0.0) |
|
|
|
Lisinopril (n=407) Incidence (discontinuation) 12 weeks |
Placebo (n=155) Incidence (discontinuation) 12 weeks |
|
|
|
|
|
|
|
Body as a Whole |
|
|
| Chest Pain |
|
3.4 (0.2) |
1.3 (0.0) |
| Abdominal Pain |
|
2.2 (0.7) | 1.9 (0.0) |
|
|
Cardiovascular |
|
|
| Hypotension |
|
4.4 (1.7) |
0.6 (0.6) |
|
|
Digestive |
|
|
| Diarrhea |
|
3.7 (0.5) | 1.9 (0.0) |
|
|
Nervous/Psychiatric |
|
|
| Dizziness |
|
11.8 (1.2) | 4.5 (1.3) |
| Headache |
|
4.4 (0.2) | 3.9 (0.0) |
|
|
Respiratory |
|
|
| Upper Respiratory Infection |
|
1.5 (0.0) | 1.3 (0.0) |
|
|
Skin |
|
|
| Rash |
|
1.7 (0.5) | 0.6 (0.6) |
| % of patients Events |
High Dose (N=1568) |
Low dose (N=1596) |
| Dizziness | 18.9 | 12.1 |
| Hypotension | 10.8 | 6.7 |
| Creatinine-increased | 9.9 | 7.0 |
| Hyperkalemia | 6.4 | 3.5 |
| NPN * increased | 9.2 | 6.5 |
| Syncope | 7.0 | 5.1 |
CLINICAL LABORATORY TEST FINDINGS
Serum Electrolytes: Hyperkalemia (See PRECAUTIONS ), hyponatremia.
Creatinine, Blood Urea Nitrogen: Minor increases in blood urea nitrogen and serum creatinine, reversible upon discontinuation of therapy, were observed in about 2% of patients with essential hypertension treated with lisinopril alone. Increases were more common in patients receiving concomitant diuretics and in patients with renal artery stenosis (See PRECAUTIONS). Reversible minor increases in blood urea nitrogen and serum creatinine were observed in approximately 11.6% of patients with heart failure on concomitant diuretic therapy. Frequently, these abnormalities resolved when the dosage of the diuretic was decreased.
Hemoglobin and Hematocrit: Small decreases in hemoglobin and hematocrit (mean decreases of approximately 0.4 g% and 1.3 vol%, respectively) occurred frequently in patients treated with lisinopril but were rarely of clinical importance in patients without some other cause of anemia. In clinical trials, less than 0.1% of patients discontinued therapy due to anemia. Hemolytic anemia has been reported; a causal relationship to lisinopril cannot be excluded.
Liver Function Tests: Rarely, elevations of liver enzymes and/or serum bilirubin have occurred (See WARNINGS, Hepatic Failure).
In hypertensive patients, 2.0% discontinued therapy due to laboratory adverse experiences, principally elevations in blood urea nitrogen (0.6%), serum creatinine (0.5%) and serum potassium (0.4%).
In the heart failure trials, 3.4% of patients discontinued therapy due to laboratory adverse experiences; 1.8% due to elevations in blood urea nitrogen and/or creatinine and 0.6% due to elevations in serum potassium.
In the myocardial infarction trial, 2.0% of patients receiving lisinopril discontinued therapy due to renal dysfunction (increasing creatinine concentration to over 3 mg/dL or a doubling or more of the baseline serum creatinine concentration); less than 1.0% of patients discontinued therapy due to other laboratory adverse experiences: 0.1% with hyperkalemia and less than 0.1% with hepatic enzyme alterations.
OVERDOSAGE
Following a single oral dose of 20 g/kg no lethality occurred in rats, and death occurred in one of 20 mice receiving the same dose. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution.
Lisinopril can be removed by hemodialysis (See WARNINGS, Anaphylactoid Reactions During Membrane Exposure).
DOSAGE AND ADMINISTRATION
Hypertension
Initial Therapy: In patients with uncomplicated essential hypertension not on diuretic therapy, the recommended initial dose is 10 mg once a day. Dosage should be adjusted according to blood pressure response. The usual dosage range is 20 to 40 mg per day administered in a single daily dose. The antihypertensive effect may diminish toward the end of the dosing interval regardless of the administered dose, but most commonly with a dose of 10 mg daily. This can be evaluated by measuring blood pressure just prior to dosing to determine whether satisfactory control is being maintained for 24 hours. If it is not, an increase in dose should be considered. Doses up to 80 mg have been used but do not appear to give greater effect. If blood pressure is not controlled with lisinopril tablet alone, a low dose of a diuretic may be added. Hydrochlorothiazide, 12.5 mg has been shown to provide an additive effect. After the addition of a diuretic, it may be possible to reduce the dose of lisinopril tablet.
Diuretic Treated Patients: In hypertensive patients who are currently being treated with a diuretic, symptomatic hypotension may occur occasionally following the initial dose of lisinopril tablet. The diuretic should be discontinued, if possible, for two to three days before beginning therapy with lisinopril tablet to reduce the likelihood of hypotension (See WARNINGS). The dosage of lisinopril tablet should be adjusted according to blood pressure response. If the patient's blood pressure is not controlled with lisinopril tablet alone, diuretic therapy may be resumed as described above.
If the diuretic cannot be discontinued, an initial dose of 5 mg should be used under medical supervision for at least two hours and until blood pressure has stabilized for at least an additional hour (See WARNINGS and PRECAUTIONS, Drug Interactions).
Concomitant administration of lisinopril with potassium supplements, potassium salt substitutes, or potassium-sparing diuretics may lead to increases of serum potassium (See PRECAUTIONS).
Dosage Adjustment in Renal Impairment: The usual dose of lisinopril tablet (10 mg) is recommended for patients with creatinine clearance Greater than 30 mL/min (serum creatinine of up to approximately 3 mg/dL). For patients with creatinine clearance Greater than or equal to 10 mL/min Less than or equal to 30 mL/min (serum creatinine Greater than or equal to 3 mg/dL), the first dose is 5 mg once daily. For patients with creatinine clearance less than 10 mL/min (usually on hemodialysis) the recommended initial dose is 2.5 mg. The dosage may be titrated upward until blood pressure is controlled or to a maximum of 40 mg daily.
| Renal Status |
Creatinine Clearance mL/min |
Initial Dose mg/day |
| Normal Renal Function to Mild Impairment | Greater than 30 | 10 |
| Moderate to Severe Impairment | Greater than or equal to 10 less than or equal to 30 |
5 |
| Dialysis Patients* | Less than 10 | 2.5†
|
HOW SUPPLIED
Lisinopril Tablets, USP are available as:
2.5 mg tablet is a white to off-white, round, biconvex uncoated tablet with ‘LUPIN" debossed on one side and "2.5" on other side. They are available as follows:
Bottles of 90 NDC 68180-512-09
Bottles of 100 NDC 68180-512-01
Bottles of 500 NDC 68180-512-02
Bottles of 1000 NDC 68180-512-03
5 mg tablet is a pink coloured, round, biconvex uncoated tablet with "5" debossed on one side and breakline on other side. They are available as follows:
Bottles of 90 NDC 68180-513-09
Bottles of 100 NDC 68180-513-01
Bottles of 500 NDC 68180-513-02
Bottles of 1000 NDC 68180-513-03
Bottles of 5000 NDC 68180-513-05
10 mg tablet is a pink coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "10" on other side. They are available as follows:
Bottles of 90 NDC 68180-514-09
Bottles of 100 NDC 68180-514-01
Bottles of 500 NDC 68180-514-02
Bottles of 1000 NDC 68180-514-03
Bottles of 5000 NDC 68180-514-05
20 mg tablet is a pink coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "20" on other side. They are available as follows:
Bottles of 90 NDC 68180-515-09
Bottles of 100 NDC 68180-515-01
Bottles of 500 NDC 68180-515-02
Bottles of 1000 NDC 68180-515-03
Bottles of 5000 NDC 68180-515-05
30 mg tablet is a red coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "30" on other side. They are available as follows:
Bottles of 90 NDC 68180-516-09
Bottles of 100 NDC 68180-516-01
Bottles of 500 NDC 68180-516-02
Bottles of 1000 NDC 68180-516-03
40 mg tablet is a yellow coloured, round, biconvex uncoated tablet with "LUPIN" debossed on one side and "40" on other side. They are available as follows:
Bottles of 90 NDC 68180-517-09
Bottles of 100 NDC 68180-517-01
Bottles of 500 NDC 68180-517-02
Bottles of 1000 NDC 68180-517-03
Bottles of 2000 NDC 68180-517-04
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States.
Manufactured by:
Lupin Limited
Goa 403 722
INDIA.
Or
Lupin Limited
Pithampur (M.P.) 454 775
INDIA.
Revised: 15th April 2011 ID#: 224652
Storage:
Store at 20º to 25ºC (68º to 77ºF)[see USP Controlled Room Temperature]. Protect from moisture, freezing and excessive heat. Dispense in a tight container.
NDC 68180-515-01 ONCE-DAILY Lisinopril Tablets USP 20 mg Each tablet contains lisinopril USP ...... 20 mg. Rx only LUPIN 100 Tablets Usual Dosage: See accompanying precribing information. Dispense in a tight container. Keep out of the reach of children, Storage: Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature]. Protect from moisture, freezing and excessive heat. Manufactured for: Lupin Pharmaceuticals. Inc. Baltimore, Maryland 21202 United States Manufactured by: Lupin Limited Goa 403 722 INDIA Code No. GOVDRUGS/654 219790 N3 68180 51501 2 LOT NO. EXP.
Hypertensa (U.S. patent pending) capsules by oral administration. A specially formulated Medical Food product, consisting of a proprietary formulation of amino acids and polyphenol ingredients in specific proportions, for the dietary management of the metabolic processes associated with hypertension. (HT). Must be administered under physician supervision.
Medical Foods
Medical Food products are often used in hospitals (e.g., for burn victims or kidney dialysis patients) and outside of a hospital setting under a physician’s care for the dietary management of diseases in patients with particular, unique or distinctive medical or metabolic needs due to their disease or condition. Congress defined "Medical Food" in the Orphan Drug Act and Amendments of 1988 as "a food which is formulated to be consumed or administered enterally [or orally] under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation." Medical Foods are complex formulated products, requiring sophisticated and exacting technology, and that are used only for a patient receiving active and ongoing medical supervision wherein the patient requires medical care on a recurring basis for, among other things, instructions on the use of the Medical Food. Hypertensa has been developed, manufactured, and labeled in accordance with both the statutory definition of a Medical Food and FDA’s regulatory labeling guidelines. Hypertensa must be used while the patient is under the ongoing care of a physician.
HYPERTENSION (HT)
HT as a Metabolic Deficiency Disease
A critical component of the definition of a Medical Food is that the product must address the distinct nutritional requirements of a particular disease or condition. FDA scientists have proposed a physiologic definition of distinctive nutritional requirements as follows: “the dietary management of patients with specific diseases requires, in some instances, the ability to meet nutritional requirements that differ substantially from the needs of healthy persons. For example, in establishing the recommended dietary allowances for general, healthy population, the Food and Nutrition Board of the Institute of Medicine National Academy of Sciences, recognized that different or distinctive physiologic requirements may exist for certain persons with "special nutritional needs arising from metabolic disorders, chronic diseases, injuries, premature birth, other medical conditions and drug therapies. Thus, the distinctive nutritional needs associated with a disease reflects the total amount needed by a healthy person to support life or maintain homeostasis, adjusted for the distinctive changes in the nutritional needs of the patient as a result of the effects of the disease process on absorption, metabolism and excretion.” It was also proposed that in patients with certain disease states who respond to nutritional therapies, a physiologic deficiency of the nutrient is assumed to exist. For example, if a patient with hypertension responds to an arginine formulation by decreasing the blood pressure, a deficiency of arginine is assumed to exist
Patients with hypertension are known to have increased nutritional requirements for arginine, choline, flavonoids, and certain antioxidants. Patients with hypertension frequently exhibit reduced plasma levels of arginine and have been shown to respond to oral administration of an arginine formulation. Research has shown that arginine reduced diets result in a fall of circulating arginine. Patients with hypertension have activation of the arginase pathway that diverts arginine from the production of nitric oxide to production of deleterious nitrogen molecules such as peroxynitrite leading to a reduced level of production of nitric oxide for a given arginine blood level. Research has also shown that a genetic predisposition can lead to increased arginine requirements in certain patients with hypertension.
Arginine is required to fully potentiate nitric oxide synthesis by the arterioles. A deficiency of arginine leads to reduced nitric oxide production by the arterioles. Low fat diets, frequently used by patients with hypertension, are usually arginine deficient. Flavonoids potentiate the production of nitric oxide by the arterioles thereby reducing blood pressure in hypertensive patients. Diets deficient in flavonoid rich foods result in inadequate arginine and flavonoid concentrations, impeding nitric oxide production in certain patients with hypertension. Provision of arginine, choline, and flavonoids with antioxidants, in the correct proportions can restore the production of beneficial nitric oxide, thereby reducing blood pressure.
PRODUCT DESCRIPTION
Primary Ingredients
Hypertensa consists of a proprietary formulation of L-Arginine, Choline Bitartrate, Whey Protein Hydrolysate, Cocoa, Cinnamon, Ginseng, L-Leucine, L-Glutamine, LHistidine, Caffeine, L-Cysteine, and Grape Seed Extract in specific proportions. These ingredients fall into the classification of Generally Recognized as Safe (GRAS) as defined by the Food and Drug Administration (FDA) (Sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act). A GRAS substance is distinguished from a food additive on the basis of the common knowledge about the safety of the substance for its intended use. The standard for an ingredient to achieve GRAS status requires not only technical demonstration of non-toxicity and safety, but also general recognition of safety through widespread usage and agreement of that safety by experts in the field. Many ingredients have been determined by the FDA to be GRAS, and are listed as such by regulation, in Volume 21 Code of Federal Regulations (CFR) Sections 182, 184, and 186.
Amino Acids
Amino Acids are the building blocks of protein and are GRAS listed as they have been safely ingested by humans for thousands of years. The formulations of the amino acids in Hypertensa are equivalent to those found in the usual human diet. Patients with hypertension may require an increased amount of certain amino acids that cannot be obtained from normal diet alone. Arginine, for example, is a conditional amino acid. The body can make arginine in the liver, but the liver produced arginine can only be used in the liver itself. Arginine is needed to produce nitric oxide (NO). NO is required to dilate the constricted blood vessels that are the cause of high blood pressure. Patients with hypertension have an increase in the enzyme, arginase that degrades arginine before it can be used to produce NO. Some patients with hypertension have a resistance to the use of arginine that is similar to the mechanism found in insulin resistance that is genetically determined. Patients with hypertension cannot obtain sufficient arginine from the diet without ingesting a prohibitively large amount of calories, particularly calories from protein.
Flavonoids
Flavonoids are a group of phytochemical compounds found in all vascular plants including fruits and vegetables. They are a part of a larger class of compounds known as polyphenols. Many of the therapeutic or health benefits of colored fruits and vegetables, cocoa, red wine, and green tea are directly related to their flavonoid content. The specially formulated flavonoids found in Hypertensa cannot be obtained from conventional foods in the necessary proportions to elicit a therapeutic response.
Other Ingredients
Hypertensa contains the following “inactive” or other ingredients, as fillers, excipients, and colorings: Tricalcium Phosphate, Gelatin, Silicone Dioxide, Vegetable Magnesium Stearate, Microcrystalline Cellulose, Chlorophyllin Copper Complex, Titanium Dioxide.
Physical Description
Hypertensa is a yellow to light brown powder. Hypertensa consists of a proprietary formulation of L-Arginine, Choline Bitartrate, Whey Protein Hydrolysate, Cocoa, Cinnamon, Ginseng, L-Leucine, L-Glutamine, L-Histidine, Caffeine, L-Cysteine, and Grape Seed Extract.
CLINICAL PHARMACOLOGY
Mechanism of Action
Hypertensa acts by restoring and maintaining the balance of Nitric Oxide (NO) in patients with hypertension.
Metabolism
The amino acids in Hypertensa are primarily absorbed by the stomach and small intestines. All cells metabolize the amino acids in Hypertensa. Circulating tryptophan, arginine and choline blood levels determine the production of serotonin, nitric oxide, and acetylcholine.
Excretion
Hypertensa is not an inhibitor of cytochrome P450 1A2, 2C9, 2C19, 2D6, or 3A4. These isoenzymes are principally responsible for 95% of all detoxification of drugs, with CYP3A4 being responsible for detoxification of roughly 50% of drugs. Amino acids do not appear to have an effect on drug metabolizing enzymes.
Uses
INDICATIONS FOR USE
Hypertensa is intended for the clinical dietary management of the metabolic processes of hypertension.
CLINICAL EXPERIENCE
Administration of Hypertensa has demonstrated significant functional improvements in reducing blood pressure in hypertensive patients, when used for the dietary management of the metabolic processes associated with hypertension. Hypertensa has no effect on normal blood pressure.
PRECAUTIONS AND CONTRAINDICATIONS
Hypertensa is contraindicated in an extremely small number of patients with hypersensitivity to any of the nutritional components of Hypertensa.
ADVERSE REACTIONS
Ingestion of L-Tryptophan, L-Arginine, or Choline at high doses of up to 15 grams daily is generally well tolerated. The most common adverse reactions of higher doses — from 15 to 30 grams daily — are nausea, abdominal cramps, and diarrhea. Hypertensa contains less than 1 gram per dose of amino acids however, some patients may experience these symptoms at lower doses. The total combined amount of amino acids in each Hypertensa capsule does not exceed 200 mg.
DRUG INTERACTIONS
Hypertensa does not directly influence the pharmacokinetics of prescription drugs. Clinical experience has shown that administration of Hypertensa may allow for
lowering the dose of co-administered drugs under physician supervision.
OVERDOSE
There is a negligible risk of overdose with Hypertensa as the total amount of amino acids in a two week supply (60 capsules) is less than 12 grams.
Overdose symptoms may include diarrhea, weakness, and nausea.
POST-MARKETING SURVEILLANCE
Post-marketing surveillance has shown no serious adverse reactions. Reported cases of mild rash and itching may have been associated with allergies to
Hypertensa flavonoid ingredients, including Cinnamon, Cocoa, and Grape Seed. These reactions were temporary, transient in nature and subsided within 24-hours.
DOSAGE AND ADMINISTRATION
Recommended Administration
For the dietary management of the metabolic processes associated with hypertension. Take two (2) capsules twice daily or as directed by physician.
As with most amino acid formulations Hypertensa should be taken without food to increase the absorption of key ingredients.
How Supplied
Hypertensa is supplied in green and white, size 0 capsules in bottles of 60 and 90 capsules.
Physician Supervision
Hypertensa is a Medical Food product available by prescription only and may be used per FDA law, and product labeling while the patient is under ongoing physician supervision.
U.S. patent pending
Manufactured by Arizona Nutritional Supplements, Inc. Chandler AZ 85225
Distributed exclusively by Physician Therapeutics LLC, a wholly owned subsidiary of Targeted Medical Pharma Inc. Los Angeles, CA.
www.ptlcentral.com
NDC: 68405-007-02
NDC: 68405-007-03
Storage
Store in a cool dry place 45-90ο F (8-32ο C) relative humidity below 50%. Hypertensa is supplied in a recyclable plastic bottle with a child-resistant cap.
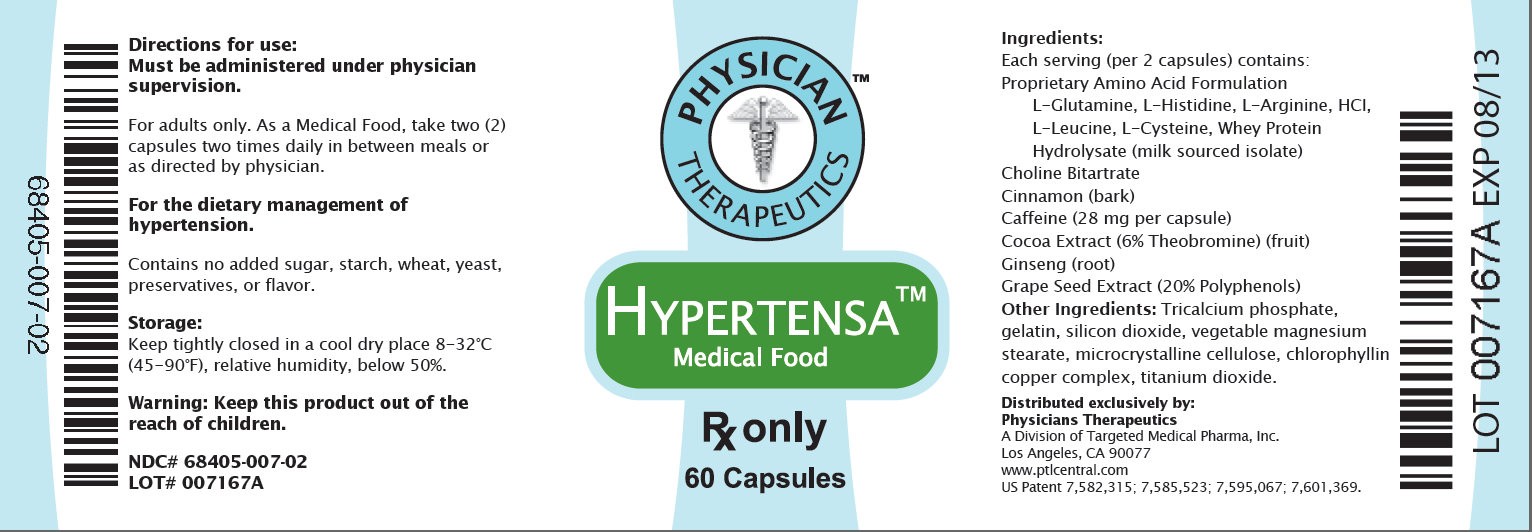
68405-007-02 Directions for use: Must be administered under physician supervision. For adults only. As a Medical Food, take two (2) capsules four times daily in between meals or as directed by physician. For the dietary management of hypertension. Contains no added sugar, starch, wheat, yeast, preservatives, flavor. Storage: Keep tightly closed in a cool dry place 8-32 degree C (45 degree -90 degree F), relative humidity, below 50%. Warning: Keep this product out of the reach of children. NDC# 68405-007-03 LOT# 007167A PHYSICIAN THERAPEUTICS HYPERTENSA Medical Food Rx only 60 Capsules Ingredients: Each serving (per 2 capsules) contains: Proprietary Amino Acid Acid Formulation L-Glutamine L-Histadine, L-Arginine, HCI, L-Leucine, L-Cysteine, Whey Protein Hydrolysate (milk sourced isolate) Choline Bitatarate, Cinnamon (bark), Caffeine (28 mg per capsule) Cocoa Extract (6% Theobromine) (fruit), Ginseng (root), Grape Seed Extract (20% Polyphenol) Other Indgredients: Tricalcium phosphate, gelatin, silicon dioxide, vegetable magnesium stearate, microcrystalline cellulose, chlorophyllin copper complex, titanium dioxide. Distributed exclusively by: Physicians Therapeutics A Division of Targeted Medical Pharma, Inc. Los Angeles, CA 90077 www.ptlcentral.com US Patent 7,582,315; 7,585,523; 7,595,067; 7,601,369. LOT 007167A EXP 08/13
A Convinience Packed Medical Food and Drug Lytensopril PHYSICIAN THERAPEUTICS > Hypertensa 60 Capsules > Lisinopril 20 mg 30 Tablets No Refills Without Physician Authorization Rx Only NDC# 68405-070-06 of this pack

LytensoprilLISINOPRIL, ARGININE KIT
| ||||||||||||||||||||||||||||||||||||||||