Lipiodol Ultra-Fluide
Lipiodol Ultra-Fluide
FULL PRESCRIBING INFORMATION: CONTENTS*
- Dear Healthcare Professional,
- Comparison Table
- SUMMARY OF PRODUCT CHARACTERISTICS
- Patient information leaflet
- Carton
- Ampoule
FULL PRESCRIBING INFORMATION
Dear Healthcare Professional,
Due to the current critical shortage of ETHIODOL®, Brand of Ethiodized Oil Injection, Guerbet is coordinating with the FDA to increase the availability of the ethyl esters of iodized fatty acids of poppy seed oil product.
Guerbet has acquired the Ethiodol® NDA from Nycomed US Inc. effective May 7, 2010 and is working with the FDA to resume manufacturing of Ethiodol in the near future to ensure continued availability for the US patients. During this interim period, Guerbet, in conjunction with the FDA, is initiating a temporary importation of LIPIODOL® ULTRA-FLUIDE, ethyl esters of iodized fatty acids of poppy seed oil, to the United States market. LIPIODOL® ULTRA-FLUIDE contains the same drug components as ETHIODOL®, Brand of Ethiodized Oil Injection, (previously manufactured and marketed in the United States by Savage Laboratories, a subsidiary of Nycomed). LIPIODOL® ULTRA-FLUIDE is manufactured in compliance with European Good Manufacturing Practice (GMP) regulations by Delpharm Tours (France) for Guerbet.
At this time, no other entity except Guerbet is authorized by the FDA to import or distribute LIPIODOL® ULTRA-FLUIDE. Any sales of LIPIODOL® ULTRA-FLUIDE ampoules from any entity other than Guerbet will be considered in violation of the Federal Food, Drug and Cosmetic Act and may be subject to enforcement action by the FDA.
Effective immediately, Guerbet will offer the following version:
|
LIPIODOL® ULTRA-FLUIDE 48% Iodine w/vol (i.e 480 mg Iodine/mL) (ethyl esters of iodized fatty acids of poppy seed oil) |
|
| 10mL glass ampoule | Authorization# 306 216.0 Box of 1 ampoule |
LIPIODOL® ULTRA-FLUIDE formulation is similar to ETHIODOL®.
The active substance of LIPIODOL ® ULTRA-FLUIDE and ETHIODOL is the same (ethyl esters of iodized fatty acids of poppy seed oil, stabilized with 1% of poppy seed oil). It is important to note that there are some key labeling differences between the international marketed LIPIODOL® ULTRA-FLUIDE and the United States marketed ETHIODOL® that you need to be aware:
- The difference in label claim is due to the unit used to express the Iodine content: the unit for ETHIODOL® is 37% Iodine w/w = weight/weight, while the unit for LIPIODOL ULTRA-FLUIDE® is 48% Iodine w/vol = weight/volume. When converting one unit to another (w/w or w/vol), the Iodine content of ETHIODOL® and LIPIODOL® ULTRA-FLUIDE are similar.
The barcode used on LIPIODOL® ULTRA-FLUIDE is an international pharmaceutical manufacturing code and will likely not be recognized by scanning systems used in the United States. Institutions should confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
For questions regarding LIPIODOL® ULTRA-FLUIDE in the United States, please contact Guerbet LLC at 1-877-729-6679 between the hours of 8 a.m. and 5 p.m. (EST), or email at info-us@guerbet-group.com.
The product comparison table below also highlights the differences between LIPIODOL® ULTRA-FLUIDE and ETHIODOL®.
Please click here for package inserts: Guerbet LIPIODOL® ULTRA-FLUIDE (Patient Information Leaflet and/or Summary of Product Characteristics) and Savage Laboratories ETHIODOL®.
- Customers can order directly from Guerbet LLC by contacting Customer Service at 1-877-729-6679 between the hours of 8 a.m. and 5 p.m. (EST).
- LIPIODOL® ULTRA-FLUIDE is not refundable and not for resale.
Guerbet will make reasonable attempts to fill your orders. Guerbet will be closely monitoring the distribution of LIPIODOL® ULTRA-FLUIDE to help manage the supply.
If you have additional questions, please contact Customer Service at 1-877-729-6679, Monday through Friday, between the hours of 8 a.m. and 5 p.m. (EST), or email customer.service-us@guerbet-group.com. This communication and updated product information is available on the Guerbet website at http://www.guerbet-us.com as well as on the FDA Drug Shortage website at http://www.fda.gov/Drugs/DrugSafety/DrugShortages/default.htm.
To report adverse events among patients administered, please call 1-877-729-6679 between the hours of 8 a.m. and 5 p.m. (EST), or email medical.liaison@guerbet-group.com.
Alternatively, any adverse events that may be related to the use of these products should be reported to the FDA's Med Watch Program by fax at 1-800-FDA-0178, by mail at Med Watch, HF-2, FDA, 5600 Fishers Lane, Rockville, MD 20852-9787, or on the Med Watch website at http:www.fda.gov/safety/medwatch/default.htm.
We urge you to contact our Medical Information Department at 1-877-729-6679 between the hours of 8 a.m. and 5 p.m. (EST), or email medical.liaison@guerbet-group.com if you have any questions about the information contained in this letter or the safe and effective use of LIPIODOL® ULTRA-FLUIDE.
Comparison Table

| LIPIODOL® ULTRA-FLUIDE (ethyl esters of iodized fatty acids of poppy seed oil) | ETHIODOL® (ethyl esters of iodized fatty acids of poppy seed oil) |
| Iodine label claim | |
| 48% w/vol Iodine (480 mg/mL) | 37% w/w Iodine (475 mg/mL) |
| Indications and contraindications | |
| See package insert Please note: see package insert sections 4.2 Method of administration, 4.3 Contraindications, and 4.4 Special warning and precautions for use. |
ETHIODOL®
is indicated for use as a radio-opaque medium for hysterosalpingography and lymphography. See package insert for contraindications. |
| Barcode | |
| Barcode use by LIPIODOL® ULTRA-FLUIDE may not register accurately in the United States scanning systems. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients. | A unit of use barcode is on individual ampoules. |
| How supplied | |
| Box of 1 ampoule NDC# 67684-1901-0 Authorization# 306 216.0 |
Box of 2 ampoules NDC# 0281-7062-37 |
| Additional information | |
| Contains a patient information leaflet | N/A |
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
LIPIODOL ULTRA-FLUIDE (480 mg I/ml), solution for injection.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Ethyl esters of iodized fatty acids of poppy seed oil * qs ad for one ampoule
* Iodine content: 48 %, i.e. 480 mg per ml.
3. PHARMACEUTICAL FORM
Solution for injection.
4. CLINICAL PARTICULARS
4.1. Therapeutic indications
In diagnostic radiology
- Lymphography
- Diagnosis of liver lesions
- Diagnosis of the spread of malignant lesions, whether hepatic or not, by selective hepatic arterial injection.
In interventional radiology
- Embolization with surgical glues
In association with surgical glues during vascular embolizations.
In endocrinology
- Prevention of iodine deficiency disorders.
This treatment should only be used when other methods of supplementation, particularly iodization of salt and/or drinking water, cannot be undertaken.
4.2. Posology and route of administration
In diagnostic radiology
- Lymphography
5 to 7 ml by intralymphatic injection only for opacification of a limb (the dose being adapted to the height of the patient), i.e. 10 to 14 ml for bilateral pedal lymphography.
- Diagnosis of liver lesions
Intra-arterial route only.
The standard dose depends on lesion size and can vary from 2 to 10 ml per patient. LIPIODOL ULTRA-FLUIDE is sometimes mixed with small amounts of water-soluble iodinated contrast agents. The CT scan should be performed 7 to 15 days after the selective injection to allow the LIPIODOL ULTRA-FLUIDE to be eliminated from the non-tumoral liver tissue.
In interventional radiology
- Embolization with surgical glues
Selective arterial catheterization only.
The dose of LIPIODOL ULTRA-FLUIDE administered at each embolization session depends on lesion size. The Lipiodol and liquid embolizing agent mixture may vary from 20 to 80% but usually consists of a 50/50 mixture.
The volume injected should not exceed 15 ml.
In endocrinology
Intramuscular injection only.
- Adults and children aged over 4 years: 1 ml every 3 years.
- Children aged under 4 years: 0.5 ml every 2 years without exceeding 3 ml.
In patients with thyroid nodules, the dose is 0.2 ml.
This product must be administered using a glass syringe.
4.3. Contraindications
In diagnostic radiology
This product must not be administered by intra-arterial, intravenous or intrathecal injection.
In the diagnosis of liver lesions, there are no particular contraindications to the examination, apart from those associated with selective arteriography.
In interventional radiology
- Embolization with surgical glues
There are no particular contraindications apart from those related to embolization, in particular the presence of portal thrombosis.
In endocrinology
This medicinal product is CONTRAINDICATED in the following situations:
- hyperthyroidism,
- large, multinodular goiters in persons aged over 45 years, due to the high risk of hyperthyroidism,
- during breast-feeding.
4.4. Special warnings and special precautions for use
This medicinal product should be used with caution in patients with a history of allergy.
Care should be taken to avoid vascular structures due to the risk of fat embolisms and not to inject the product into an area affected by haemorrhage or trauma, except in the specific cases described below:
In diagnostic radiology
- lymphography
Intralymphatic injection only.
After chemotherapy or radiotherapy, the lymph nodes decrease substantially in size and only retain small amounts of contrast agent. The dose injected must therefore be reduced.
Overdoses can be avoided by radiological or radioscopic monitoring during the injection.
In subjects with cardiorespiratory failure, particularly elderly patients, the doses should be adapted or the examination itself cancelled, since a portion of the product will temporarily embolize the pulmonary capillaries.
Any thyroid explorations should be performed before the radiological examination, as lymphography saturates the thyroid with iodine for several months.
- Diagnosis of liver lesions
Intra-arterial injection only
Special care should be taken in cirrhotic patients.
The examination should only be performed if it contributes to therapeutic decision-making.
In interventional radiology
- Embolization with surgical glues
Selective arterial catheterization only
Vascular embolization with liquid agents is a complex and delicate technique which should only be performed by trained physicians in an appropriate medicosurgical setting.
A premature polymerisation reaction may exceptionally occur between Lipiodol Ultra-Fluide and certain glues or batches of glues. Prior to any use of new batches of Lipiodol Ultra-Fluide or glue, it is mandatory to verify in vitro the compatibility between the glue used and Lipiodol Ultra-Fluide.
In endocrinology
Intramuscular injection only.
Do not associate other methods of iodine supplementation. The risk of thyrotoxicosis is increased if the treatment is associated with other methods of iodine supplementation, particularly iodization of foodstuffs.
Because of the risk of hyperthyroidism:
- it is advisable to avoid administering this treatment to subjects over the age of 45 years,
- and to reduce the dose in patients with thyroid nodules (see Posology and Route of Administration).
4.5. Interactions with other medicinal products and other forms of interaction
Associations requiring precautions for use
* Beta-blockers
In the event of shock or hypotension due to iodinated contrast agents, reduction of compensatory cardiovascular reactions by treatment with beta-blockers.
Treatment with beta-blockers should be stopped, whenever possible, before the radiological investigation. When continuation of treatment is essential, adequate resuscitation equipment must be available.
* Diuretics
In the event of dehydration provoked by diuretics, the risk of acute renal failure is increased, especially when high doses of iodinated contrast agents are used.
Precautions for use: re-hydration before administration of the iodinated contrast agent.
* Metformin
Lactic acidosis triggered by impaired renal function induced by the radiological investigation in diabetic patients.
Treatment with metformin must be suspended 48 hours before the investigation and only restarted 2 days after the radiological examination.
Associations to be taken into account
* Interleukin II
The risk of developing a reaction to the contrast agents is increased in the event of previous treatment with interleukin II (IV route): skin rash or, more rarely, hypotension, oliguria, or even renal failure.
4.6. Pregnancy and lactation
In endocrinology
It appears that in populations with moderate to severe iodine deficiency, it can be beneficial for pregnant women to receive iodine supplementation.
This medicinal product is highly concentrated in the maternal milk. Due to the risk of hypothyroidism in neonates, Lipiodol is contraindicated during breast-feeding.
4.7. Effects on ability to drive and use machines
Not applicable.
4.8. Undesirable effects
Allergic-like reactions may occur.
In diagnostic radiology
- Lymphography
A fever of 38-39°C may be observed in the 24 hours following the examination.
A transient lipiodol miliary is often observed on radiological images, particularly following a high or inappropriate dose. This usually remains clinically silent. In exceptional cases, pulmonary or cerebral embolism may be observed.
Spinal cord accidents are rare.
- Diagnosis of liver lesions
Fever is often observed. Other rarer complications may occur: nausea, vomiting and diarrhoea.
In interventional radiology
- Embolization with surgical glues
No undesirable effects directly related to LIPIODOL ULTRA-FLUIDE have been specifically described.
In endocrinology
Hyperthyroidism (see Precautions for use).
4.9. Overdose
In radiology
Following intralymphatic injection, cardiorespiratory and central venous complications are proportional to the dose of LIPIODOL ULTRA-FLUIDE injected.
5. PHARMACOLOGICAL PROPERTIES
5.1. Pharmacodynamic properties
NON-WATER-SOLUBLE CONTRAST AGENTS, Code ATC: V08AD01
(V: Other)
5.2. Pharmacokinetic properties
After intralymphatic injection
Lipiodol is released into the blood, taken up by the liver and lungs where the oily droplets are degraded in the pulmonary alveoli, spleen and adipose tissue.
After being taken up by the tissues and storage organs, reabsorption of Lipiodol occurs over a period lasting from a few days to several months or years. This is continuous and regular and the presence of iodides in the urine can be detected as long as contrast material is visible on the images.
After intramuscular injection
A portion of the oil accumulates in the muscle and adjacent tissues. Another portion is deiodinated via the metabolic route, the iodine being used to compensate for the iodine losses of the thyroid.
Urinary iodine excretion is massive and occurs rapidly (within the first few hours after the injection) but continues over the following months.
Urinary iodine excretion falls to 50 μg/day in adults within 3 to 5 years.
After selective intra-arterial injection
The iodine is eliminated mainly in the urine. The iodinated contrast agent is significantly more concentrated in the tumour than in the surrounding tissue, especially in the case of hepatocellular carcinomas.
5.3. Preclinical safety data
Not applicable.
6. PHARMACEUTICAL PARTICULARS
6.1. Incompatibilities
Plastic is not suitable for the storage of LIPIODOL ULTRA-FLUIDE. In the absence of any specific compatibility studies, plastic containers and syringes should not be used.
6.2. Shelf-life
3 years.
6.3. Special precautions for storage
Store protected from light.
6.4. Nature and contents of container
5 ml or 10 ml type I glass ampoule.
7. PRESENTATION AND MARKETING AUTHORISATION NUMBERS
306 217.7 - 5 ml glass ampoule, box of 4
306 216.0 - 10 ml glass ampoule, box of 1
560 350-7 - 5 ml glass ampoule, box of 100
560 351-3 - 10 ml glass ampoule, box of 50
8. LEGAL STATUS
Not applicable.
9. MARKETING AUTHORISATION HOLDER
Guerbet
BP 57400
F-95943 Roissy CdG cedex
FRANCE
10. DATE OF REVISION
November 3, 2005
Patient information leaflet

Carton
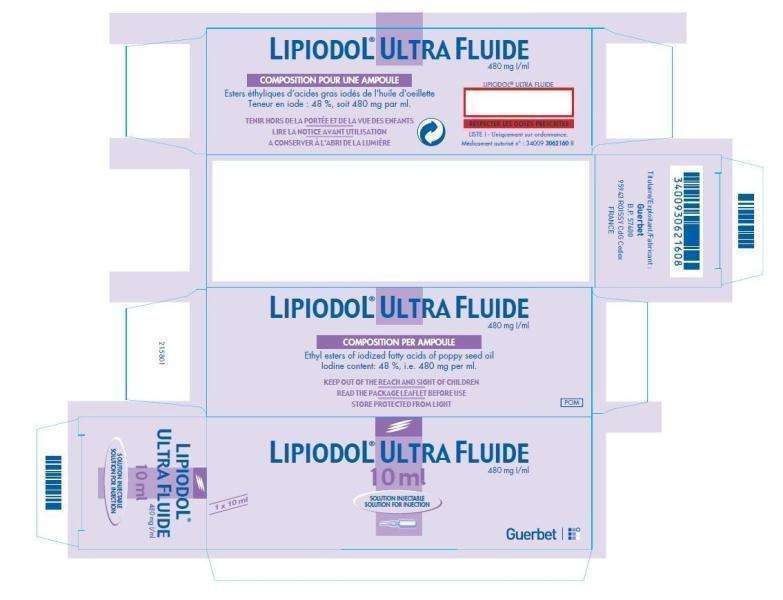
Ampoule
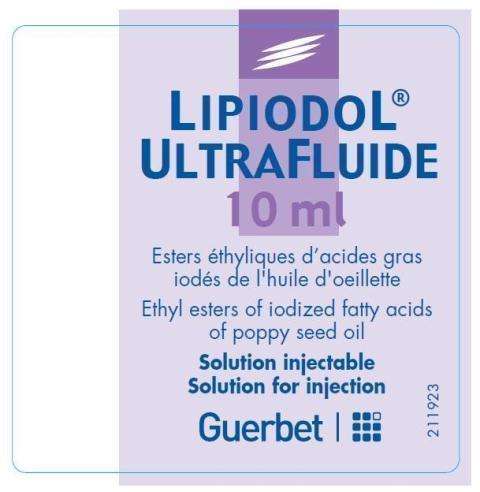
Lipiodol Ultra-FluideEthiodized Oil INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||