Librax
Valeant Pharmaceuticals International
LIBRAX (chlordiazepoxide HCl) (clidinium bromide) CAPSULES
FULL PRESCRIBING INFORMATION: CONTENTS*
- LIBRAX DESCRIPTION
- ANIMAL PHARMACOLOGY
- LIBRAX CONTRAINDICATIONS
- WARNINGS
- OVERDOSAGE
- PRECAUTIONS
- LIBRAX ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- LIBRAX DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Label
FULL PRESCRIBING INFORMATION
to control emotional
and somatic factors
in gastrointestinal disorders
LIBRAX DESCRIPTION
Librax combines in a single capsule formulation the antianxiety action of chlordiazepoxide hydrochloride and the anticholinergic/spasmolytic effects of clidinium bromide, both exclusive developments of Roche research.
Each Librax capsule contains 5 mg chlordiazepoxide hydrochloride and 2.5 mg clidinium bromide. Each capsule also contains corn starch, lactose and talc. Gelatin capsule shells may contain methyl and propyl parabens and potassium sorbate, with the following dye systems: D&C Yellow No. 10 and either FD&C Blue No. 1 or FD&C Green No. 3.
Chlordiazepoxide hydrochloride is a versatile, therapeutic agent of proven value for the relief of anxiety and tension. It is indicated when anxiety, tension or apprehension are significant components of the clinical profile. It is among the safer of the effective psychopharmacologic compounds.
Chlordiazepoxide hydrochloride is 7-chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazepine 4-oxide hydrochloride. A colorless, crystalline substance, it is soluble in water. It is unstable in solution and the powder must be protected from light. The molecular weight is 336.22. The structural formula of chlordiazepoxide hydrochloride is as follows:
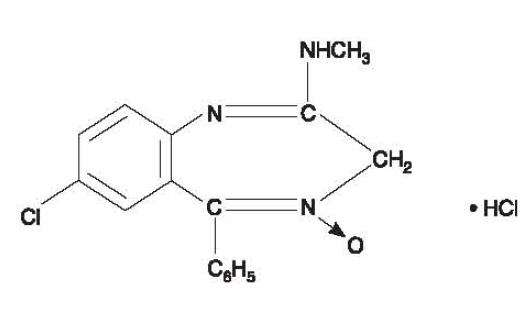
Clidinium bromide is a synthetic anticholinergic agent which has been shown in experimental and clinical studies to have a pronounced antispasmodic and antisecretory effect on the gastrointestinal tract. Structurally clidinium bromide is:
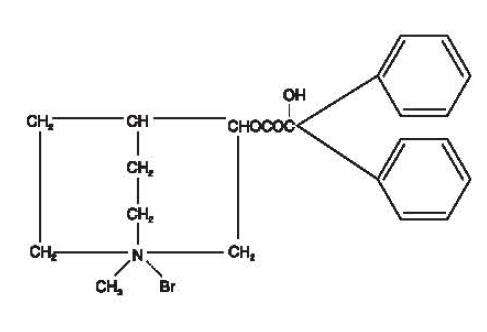
ANIMAL PHARMACOLOGY
Chlordiazepoxide hydrochloride has been studied extensively in many species of animals and these studies are suggestive of action on the limbic system of the brain, which recent evidence indicates is involved in emotional responses.
Hostile monkeys were made tame by oral drug doses which did not cause sedation. Chlordiazepoxide hydrochloride revealed a "taming" action with the elimination of fear and aggression. The taming effect of chlordiazepoxide hydrochloride was further demonstrated in rats made vicious by lesions in the septal area of the brain. The drug dosage which effectively blocked the vicious reaction was well below the dose which caused sedation in these animals.
The oral LD50 of single doses of chlordiazepoxide hydrochloride, calculated according to the method of Miller and Tainter, is 720 ± 51 mg/kg as determined in mice observed over a period of 5 days following dosage.
Clidinium bromide is an effective anticholinergic agent with activity approximating that of atropine sulfate against acetylcholine-induced spasms in isolated intestinal strips. On oral administration in mice it proved an effective antisialagogue in preventing pilocarpine-induced salivation. Spontaneous intestinal motility in both rats and dogs is reduced following oral dosing with 0.1 to 0.25 mg/kg. Potent cholinergic ganglionic blocking effects (vagal) are produced with intravenous usage in anesthetized dogs.
Oral doses of 2.5 mg/kg to dogs produced signs of nasal dryness and slight pupillary dilation. In two other species, monkeys and rabbits, doses of 5 mg/kg, po, given three times daily for 5 days did not produce apparent secretory or visual changes.
The oral LD50 of single doses of clidinium bromide is 860 ± 57 mg/kg as determined in mice observed over a period of 5 days following dosage; the calculations were made according to the method of Miller and Tainter.
Effects on Reproduction
Reproduction studies in rats fed chlordiazepoxide hydrochloride, 10, 20 and 80 mg/kg daily, and bred through one or two matings showed no congenital anomalies, nor were there adverse effects on lactation of the dams or growth of the newborn. However, in another study at 100 mg/kg daily there was noted a significant decrease in the fertilization rate and a marked decrease in the viability and body weight of offspring which may be attributable to sedative activity, thus resulting in lack of interest in mating and lessened maternal nursing and care of the young. One neonate in each of the first and second matings in the rat reproduction study at the 100 mg/kg dose exhibited major skeletal defects. Further studies are in progress to determine the significance of these findings.
Two series of reproduction experiments with clidinium bromide were carried out in rats, employing dosages of 2.5 and 10 mg/kg daily in each experiment. In the first experiment clidinium bromide was administered for a 9-week interval prior to mating; no untoward effect on fertilization or gestation was noted. The offspring were taken by caesarean section and did not show a significant incidence of congenital anomalies when compared to control animals. In the second experiment adult animals were given clidinium bromide for 10 days prior to and through two mating cycles. No significant effects were observed on fertility, gestation, viability of offspring or lactation, as compared to control animals, nor was there a significant incidence of congenital anomalies in the offspring derived from these experiments.
A reproduction study of Librax was carried out in rats through two successive matings. Oral daily doses were administered in two concentrations: 2.5 mg/kg chlordiazepoxide hydrochloride with 1.25 mg/kg clidinium bromide or 25 mg/kg chlordiazepoxide hydrochloride with 12.5 mg/kg clidinium bromide. In the first mating no significant differences were noted between the control or the treated groups, with the exception of a slight decrease in the number of animals surviving during lactation among those receiving the highest dosage. As with all anticholinergic drugs, an inhibiting effect on lactation may occur. In the second mating similar results were obtained except for a slight decrease in the number of pregnant females and in the percentage of offspring surviving until weaning. No congenital anomalies were observed in both matings in either the control or treated groups. Additional animal reproduction studies are in progress.
INDICATIONS AND USAGE
Based on a review of this drug by the National Academy of Sciences — National Research Council and/or other information, FDA has classified the indications as follows:
"Possibly" effective: as adjunctive therapy in the treatment of peptic ulcer and in the treatment of the irritable bowel syndrome (irritable colon, spastic colon, mucous colitis) and acute enterocolitis.
Final classification of the less-than-effective indications requires further investigation.
LIBRAX CONTRAINDICATIONS
Librax is contraindicated in the presence of glaucoma (since the anticholinergic component may produce some degree of mydriasis) and in patients with prostatic hypertrophy and benign bladder neck obstruction. It is contraindicated in patients with known hypersensitivity to chlordiazepoxide hydrochloride and/or clidinium bromide.
WARNINGS
As in the case of other preparations containing CNS-acting drugs, patients receiving Librax should be cautioned about possible combined effects with alcohol and other CNS depressants. For the same reason, they should be cautioned against hazardous occupations requiring complete mental alertness such as operating machinery or driving a motor vehicle.
Usage In Pregnancy
An Increased risk of congenital malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam and meprobamate) during the first trimester of pregnancy has been suggested In several studies. Because use of these drugs Is rarely a matter of urgency, their use during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of Institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physicians about the desirability of discontinuing the drug.
As with all anticholinergic drugs, an inhibiting effect on lactation may occur (see Animal Pharmacology).
OVERDOSAGE
Manifestations of chlordiazepoxide hydrochloride overdosage include somnolence, confusion, coma and diminished reflexes. Respiration, pulse and blood pressure should be monitored, as in all cases of drug overdosage, although, in general, these effects have been minimal following chlordiazepoxide hydrochloride overdosage.
While the signs and symptoms of Librax overdosage may be produced by either of its components, usually such symptoms will be overshadowed by the anticholinergic actions of clidinium bromide. The symptoms of overdosage of clidinium bromide are excessive dryness of mouth, blurring of vision, urinary hesitancy and constipation.
General supportive measures should be employed, along with immediate gastric lavage. Administer physostigmine 0.5 to 2 mg at a rate of no more than 1 mg per minute. This may be repeated in 1 to 4 mg doses if arrhythmias, convulsions or deep coma recur. Intravenous fluids should be administered and an adequate airway maintained. Hypotension may be combated by the use of levarterenol or metaraminol. Methylphenidate or caffeine and sodium benzoate may be given to combat CNS-depressive effects. Dialysis is of limited value. Should excitation occur, barbiturates should not be used. As with the management of intentional overdosage with any drug, it should be borne in mind that multiple agents may have been ingested.
Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of benzodiazepines (see DRUG ABUSE AND DEPENDENCE section).
PRECAUTIONS
In debilitated patients, it is recommended that the dosage be limited to the smallest effective amount to preclude the development of ataxia, oversedation or confusion (not more than 2 Librax capsules per day initially, to be increased gradually as needed and tolerated). In general, the concomitant administration of Librax and other psychotropic agents is not recommended. If such combination therapy seems indicated, careful consideration should be given to the pharmacology of the agents to be employed — particularly when the known potentiating compounds such as the MAO inhibitors and phenothiazines are to be used. The usual precautions in treating patients with impaired renal or hepatic function should be observed.
Paradoxical reactions to chlordiazepoxide hydrochloride, eg, excitement, stimulation and acute rage, have been reported in psychiatric patients and should be watched for during Librax therapy. The usual precautions are indicated when chlordiazepoxide hydrochloride is used in the treatment of anxiety states where there is any evidence of impending depression; it should be borne in mind that suicidal tendencies may be present and protective measures may be necessary. Although clinical studies have not established a cause and effect relationship, physicians should be aware that variable effects on blood coagulation have been reported very rarely in patients receiving oral anticoagulants and chlordiazepoxide hydrochloride.
Information for Patients
To assure the safe and effective use of benzodiazepines, patients should be informed that, since benzodiazepines may produce psychological and physical dependence, it is advisable that they consult with their physician before either increasing the dose or abruptly discontinuing this drug.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Geriatric subjects may be particularly prone to experiencing drowsiness, ataxia and confusion while receiving Librax. These effects can usually be avoided with proper dosage adjustment, although they have occasionally been observed even at the lower dosage ranges. Dosing in geriatric subjects should be initiated cautiously (no more than 2 capsules per day) and increased gradually if needed and tolerated (see DOSAGE AND ADMINISTRATION). Librax is contraindicated in the presence of glaucoma, prostatic hypertrophy and benign bladder neck obstruction (see CONTRAINDICATIONS).
LIBRAX ADVERSE REACTIONS
No side effects or manifestations not seen with either compound alone have been reported with the administration of Librax. However, since Librax contains chlordiazepoxide hydrochloride and clidinium bromide, the possibility of untoward effects which may be seen with either of these two compounds cannot be excluded.
When chlordiazepoxide hydrochloride has been used alone the necessity of discontinuing therapy because of undesirable effects has been rare. Drowsiness, ataxia and confusion have been reported in some patients — particularly the elderly and debilitated. While these effects can be avoided in almost all instances by proper dosage adjustment, they have occasionally been observed at the lower dosage ranges. In a few instances syncope has been reported.
Other adverse reactions reported during therapy with chlordiazepoxide hydrochloride include isolated instances of skin eruptions, edema, minor menstrual irregularities, nausea and constipation, extrapyramidal symptoms, as well as increased and decreased libido. Such side effects have been infrequent and are generally controlled with reduction of dosage. Changes in EEG patterns (low-voltage fast activity) have been observed in patients during and after chlordiazepoxide hydrochloride treatment.
Blood dyscrasias, including agranulocytosis, jaundice and hepatic dysfunction have occasionally been reported during therapy with chlordiazepoxide hydrochloride. When chlordiazepoxide hydrochloride treatment is protracted, periodic blood counts and liver function tests are advisable.
Adverse effects reported with use of Librax are those typical of anticolinergic agents, ie, dryness of the mouth, blurring of vision, urinary hesitancy and constipation. Constipation has occurred most often when Librax therapy has been combined with other spasmolytic agents and/or a low residue diet.
DRUG ABUSE AND DEPENDENCE
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (convulsions, tremor, abdominal and muscle cramps, vomiting and sweating), have occurred following abrupt discontinuance of chlordiazepoxide. The more severe withdrawal symptoms have usually been limited to those patients who had received excessive doses over an extended period of time. Generally milder withdrawal symptoms (eg, dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving chlordiazepoxide or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
LIBRAX DOSAGE AND ADMINISTRATION
Because of the varied individual responses to tranquilizers and anticholinergics, the optimum dosage of Librax varies with the diagnosis and response of the individual patient. The dosage, therefore, should be individualized for maximum beneficial effects. The usual maintenance dose is 1 or 2 capsules, 3 or 4 times a day administered before meals and at bedtime.
Geriatric Dosing
Dosage should be limited to the smallest effective amount to preclude the development of ataxia, oversedation or confusion. The initial dose should not exceed 2 Librax capsules per day, to be increased gradually as needed and tolerated.
HOW SUPPLIED
Librax is available in green capsules, each containing 5 mg chlordiazepoxide hydrochloride and 2.5 mg clidinium bromide — bottles of 100 (NDC 0187-4100-10).
Manufactured by:
Legacy Pharmaceuticals Puerto Rico, LLC
Humacao, Puerto Rico 00791
Manufactured for:
VALEANT®
Pharmaceuticals North America
One Enterprise, Aliso Viejo, CA 92656 USA
02-4100-EX-00
Rev. March 09
PRINCIPAL DISPLAY PANEL - 100 Capsule Bottle Label
NDC 0187-4100-10
Rx Only
Librax®
(chlordiazepoxide HCl)
(clidinium bromide)
Each capsule
contains 5 mg
chlordiazepoxide
HCl and 2.5 mg
clidinium bromide.
VALEANT®
100 Capsules

LibraxChlordiazepoxide Hydrochloride and Clidinium Bromide CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||