Levothyroxine Sodium
FULL PRESCRIBING INFORMATION: CONTENTS*
- LEVOTHYROXINE SODIUM DESCRIPTION
- INACTIVE INGREDIENT
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- LEVOTHYROXINE SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LEVOTHYROXINE SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
LEVOTHYROXINE SODIUM DESCRIPTION
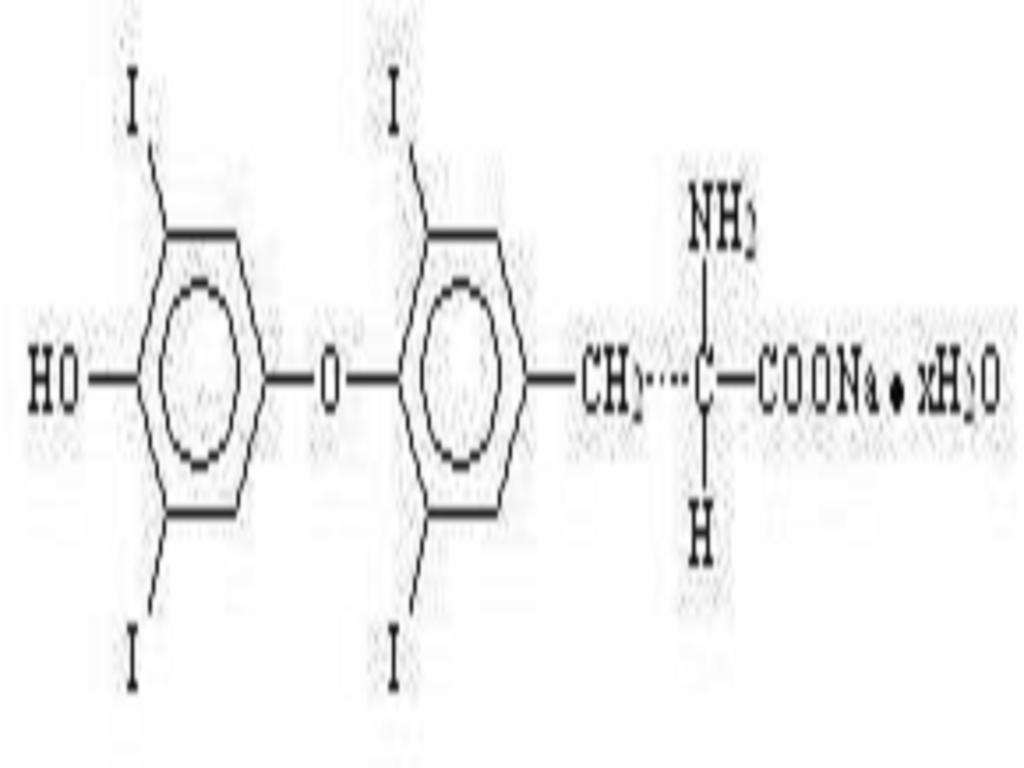
INACTIVE INGREDIENT
CLINICAL PHARMACOLOGY
INDICATIONS AND USAGE, PRECAUTIONSDOSAGE AND ADMINISTRATION
PHARMACOKINETICS
PRECAUTIONS, Drug InteractionsDrug-Food InteractionsPRECAUTIONS, Drug InteractionsDrug-Laboratory Test InteractionsPRECAUTIONS, Pregnancy
Table 1
Table 1: Pharmacokinetic Parameters of Thyroid Hormones in Euthyroid Patients
INDICATIONS & USAGE
WARNINGSPRECAUTIONSWARNINGSPRECAUTIONSWARNINGSPRECAUTIONS
LEVOTHYROXINE SODIUM CONTRAINDICATIONS
PRECAUTIONSDESCRIPTION, Inactive IngredientsWARNINGS
CONTRAINDICATIONS
PRECAUTIONS
GeneralDrug Interactions
WARNINGSPRECAUTIONS, Geriatric UseDOSAGE AND ADMINISTRATION
WARNINGSCONTRAINDICATIONS
Associated endocrine disorders
PRECAUTIONS, Autoimmune polyglandular syndrome
Autoimmune polyglandular syndrome
PRECAUTIONS, Drug Interactions
Other associated medical conditions
INFORMATION FOR PATIENTS
LABORATORY TESTS
PRECAUTIONS, Drug InteractionsDrug-Laboratory Test Interactions
WARNINGSPRECAUTIONSDOSAGE AND ADMINISTRATION
PRECAUTIONS, Pediatric UseDOSAGE AND ADMINISTRATION
DRUG INTERACTIONS
aboveTable 2
Table 2
Drug-Food Interactions
Drug-Laboratory Test Interactions
Table 2
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
DOSAGE AND ADMINISTRATIONTable 3PRECAUTIONS, Laboratory Tests
PRECAUTIONS
PRECAUTIONS, Laboratory TestsDOSAGE and ADMINISTRATION
GERIATRIC USE
WARNINGSPRECAUTIONSDOSAGE AND ADMINISTRATIONLEVOTHYROXINE SODIUM ADVERSE REACTIONS
PRECAUTIONSOVERDOSAGEOVERDOSAGE
PRECAUTIONSADVERSE REACTIONSDOSAGE & ADMINISTRATION
WARNINGSPRECAUTIONSPRECAUTIONS, Laboratory Tests
PRECAUTIONS, Drug InteractionsInformation for Patients
PRECAUTIONS
WARNINGSPRECAUTIONS, Laboratory Tests
PRECAUTIONS, Laboratory Tests
PRECAUTIONS, Pediatric Use
PRECAUTIONS , Drug-Food Interactions
Table 3
PRECAUTIONS, Laboratory TestsPediatric UsePregnancy
CONTRAINDICATIONSWARNINGSPRECAUTIONS
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Levothyroxine SodiumLevothyroxine Sodium TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!