Levetiracetam
FULL PRESCRIBING INFORMATION: CONTENTS*
- LEVETIRACETAM DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- USE IN SPECIFIC POPULATIONS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- LEVETIRACETAM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LEVETIRACETAM ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
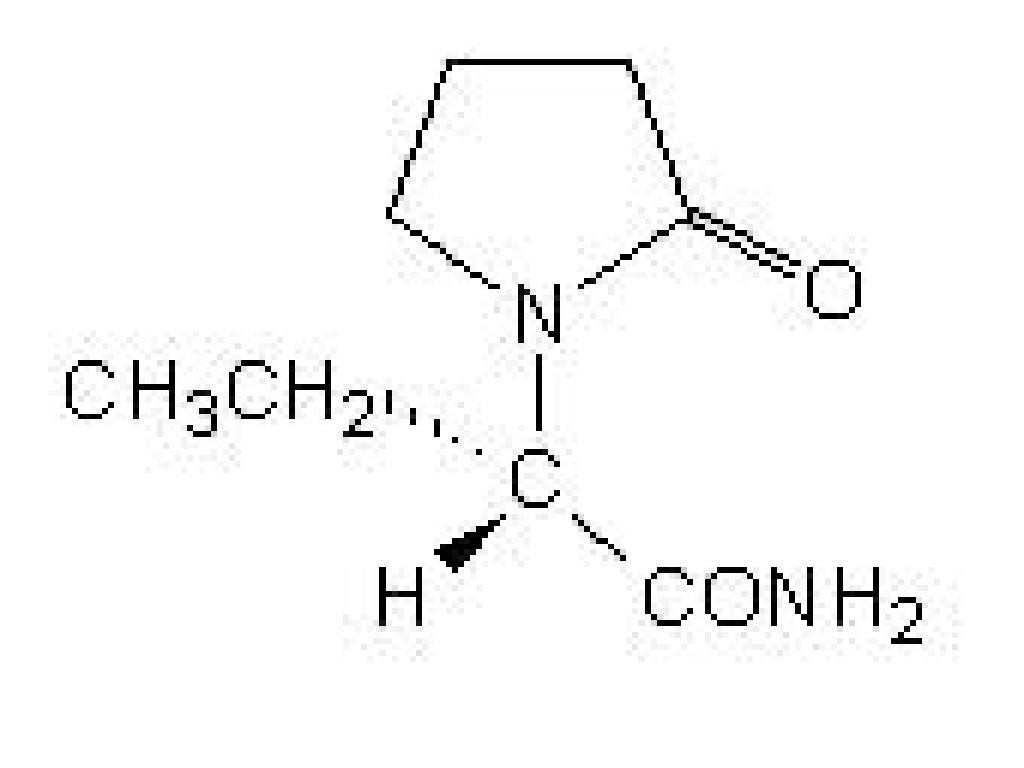
LEVETIRACETAM DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionPHARMACOKINETICS
Overview
Absorption and Distribution
Metabolism
Elimination
Special Populations, Renal ImpairmentDOSAGE AND ADMINISTRATION, Adult Patients with Impaired Renal Function)
Pharmacokinetic Interactions
PRECAUTIONS, Drug Interactions)
USE IN SPECIFIC POPULATIONS
ElderlyPediatric Patients
PRECAUTIONS, Drug Interactions
Gender
Levetiracetam Cmax and AUC were 20% higher in women (N=11) compared to men (N=12). However, clearances adjusted for body weight were comparable.
Race
Formal pharmacokinetic studies of the effects of race have not been conducted. Cross study comparisons involving Caucasians (N=12) and Asians (N=12), however, show that pharmacokinetics of levetiracetam were comparable between the two races. Because levetiracetam is primarily renally excreted and there are no important racial differences in creatinine clearance, pharmacokinetic differences due to race are not expected.
Renal Impairment
The disposition of levetiracetam was studied in adult subjects with varying degrees of renal function. Total body clearance of levetiracetam is reduced in patients with impaired renal function by 40% in the mild group (CLcr = 50 to 80 mL/min), 50% in the moderate group (CLcr = 30 to 50 mL/min) and 60% in the severe renal impairment group (CLcr <30 mL/min). Clearance of levetiracetam is correlated with creatinine clearance.
In anuric (end stage renal disease) patients, the total body clearance decreased 70% compared to normal subjects (CLcr >80 mL/min). Approximately 50% of the pool of levetiracetam in the body is removed during a standard 4-hour hemodialysis procedure.
Dosage should be reduced in patients with impaired renal function receiving levetiracetam, and supplemental doses should be given to patients after dialysis (seePRECAUTIONSandDOSAGE AND ADMINISTRATION, Adult Patients with Impaired Renal Function).
Hepatic Impairment
In subjects with mild (Child-Pugh A) to moderate (Child-Pugh B) hepatic impairment, the pharmacokinetics of levetiracetam were unchanged. In patients with severe hepatic impairment (Child-C), total body clearance was 50% that of normal subjects, but decreased renal clearance accounted for most of the decrease. No dose adjustment is needed for patients with hepatic impairment.
CLINICAL STUDIES
Effectiveness in Partial Onset Seizures in Adults with Epilepsy
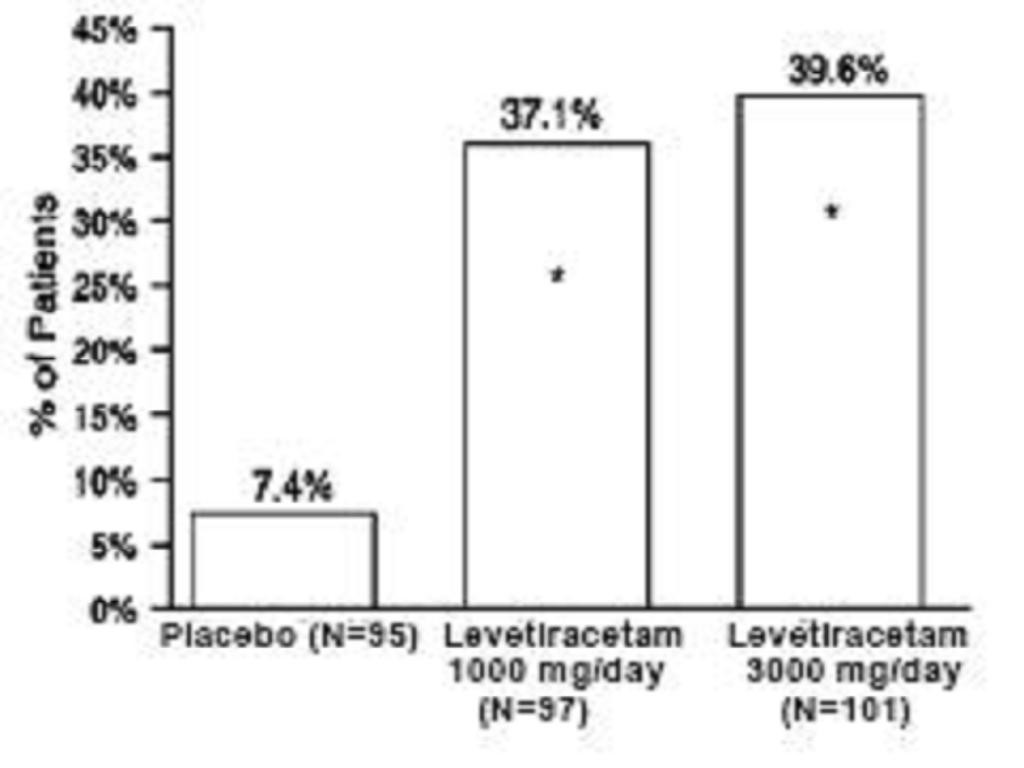
Study 1
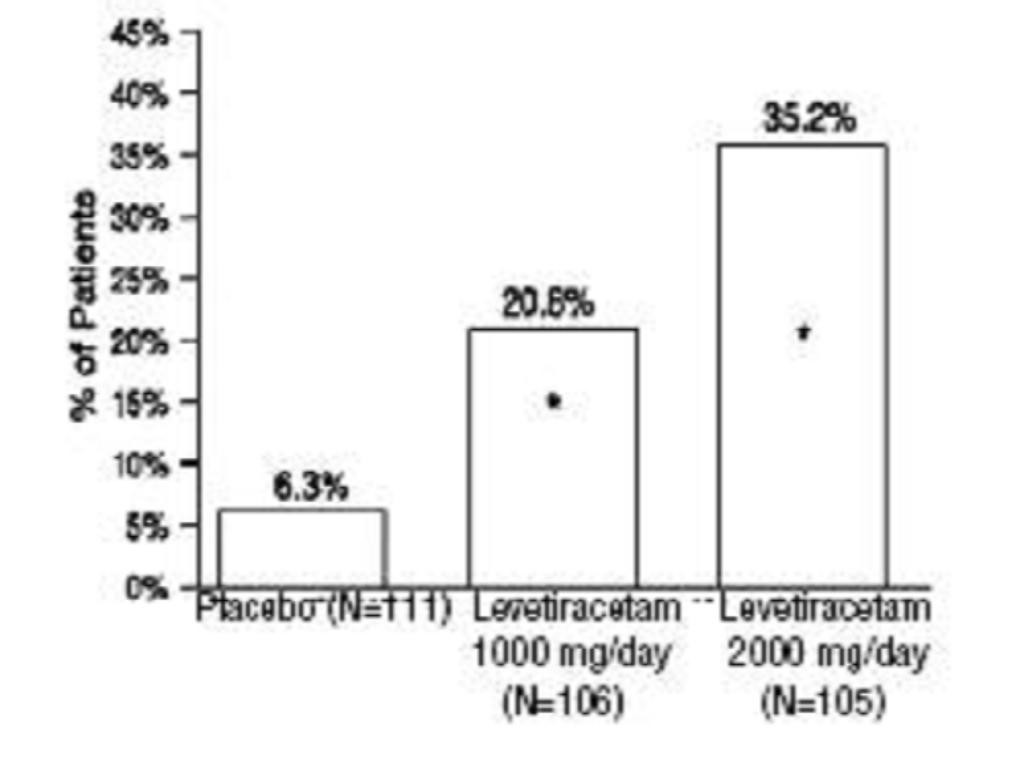
Study2
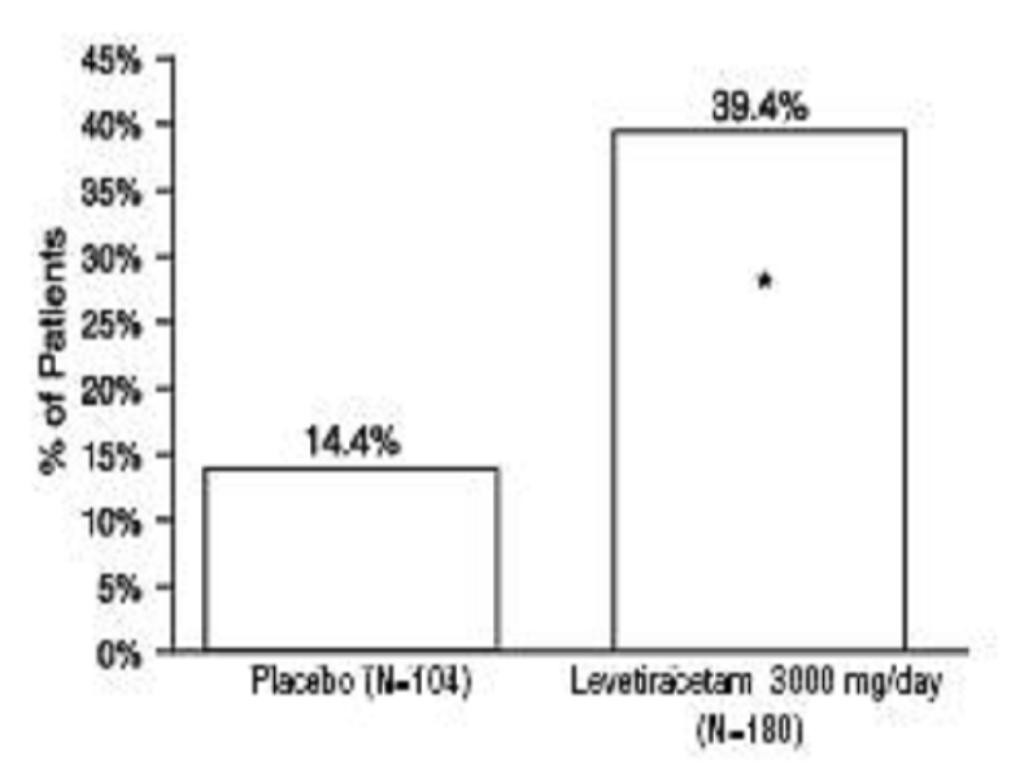
Study3
Effectiveness in Partial Onset Seizures in Pediatric Patients With Epilepsy
<%0A%09%09%09%09%09%09%09>
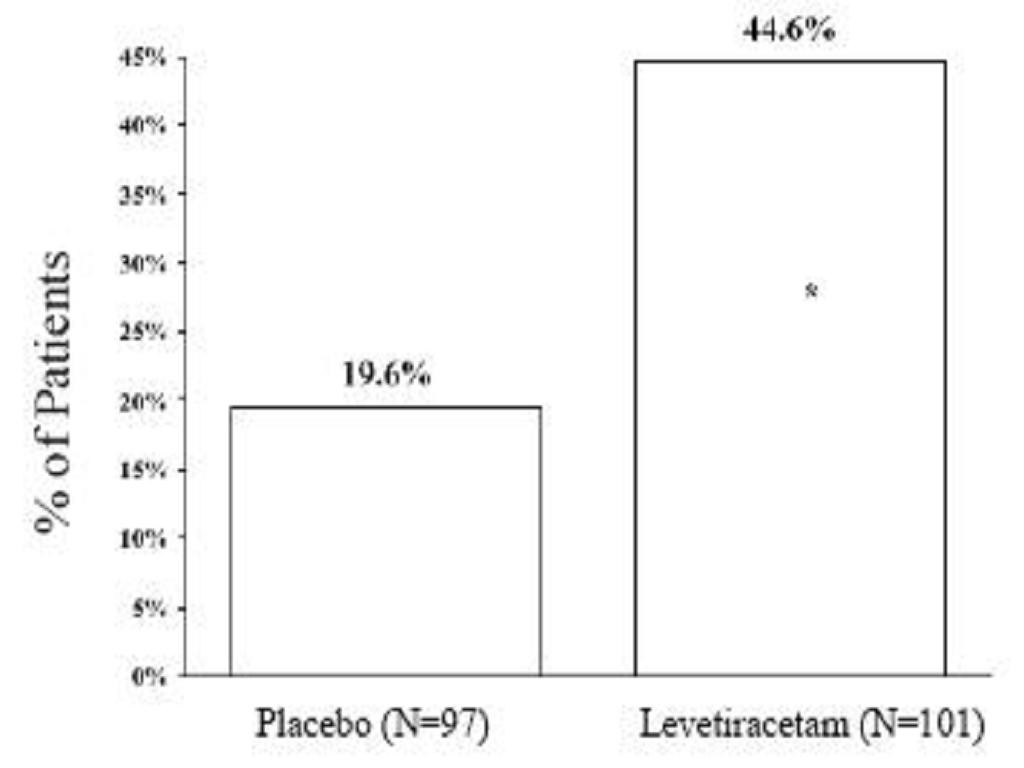
Effectiveness in Myoclonic Seizures in PatientsYears of Age with Juvenile Myoclonic Epilepsy (JME)
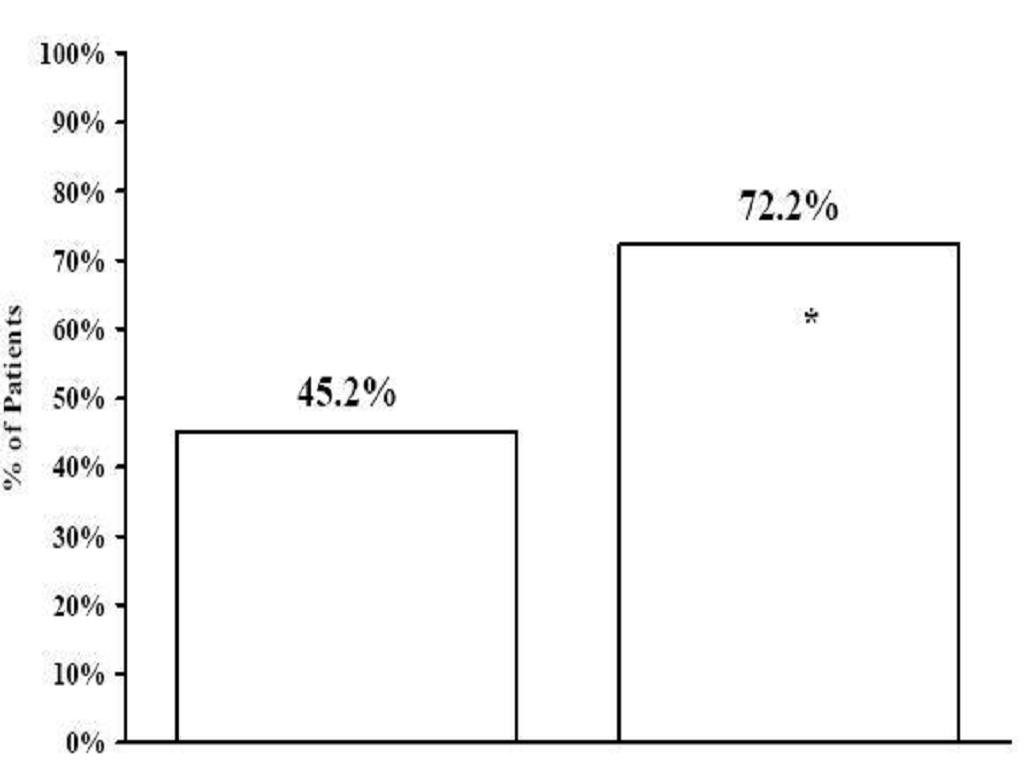
Effectiveness for Primary Generalized Tonic-Clonic Seizures in PatientsYears of Age
INDICATIONS & USAGE
LEVETIRACETAM CONTRAINDICATIONS
WARNINGS
Suicidal Behavior and IdeationNeuropsychiatric Adverse Events
Partial Onset Seizures
Adults
Pediatric Patients
Myoclonic Seizures
Primary Generalized Tonic-Clonic Seizures
Withdrawal Seizures
PRECAUTIONS
Hematologic Abnormalities
Partial Onset Seizures
Adults
Pediatric Patients
Juvenile Myoclonic Epilepsy
Hepatic Abnormalities
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
Drug-Drug Interactions between Levetiracetam and Other Antiepileptic Drugs (AEDs)Phenytoin
Phenytoin
Valproate
Effect of AEDs in Pediatric Patients
Other Drug Interactions
Oral Contraceptives
Digoxin
Warfarin
Probenecid
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Impairment of Fertility
PREGNANCY
Pregnancy Category CPregnancy Registries
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
Use in Patients with Impaired Renal Function
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION, Adult Patients with Impaired Renal Function).
LEVETIRACETAM ADVERSE REACTIONS
Partial Onset Seizures
Myoclonic Seizures
Primary Generalized Tonic-Clonic Seizures
Time Course of Onset of Adverse Events for Partial Onset Seizures
Discontinuation or Dose Reduction in Well-Controlled Clinical Studies
Partial Onset Seizures
Myoclonic Seizures
Primary Generalized Tonic-Clonic Seizures
Comparison of Gender, Age and Race
Postmarketing Experience
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
Signs, Symptoms and Laboratory Findings Of Acute Overdosage in HumansTreatment or Management of Overdose
Hemodialysis
DOSAGE & ADMINISTRATION
Partial Onset Seizures
Adults 16 Years and Older
CLINICAL STUDIES
Pediatric Patients Ages 4 to <16 Years
Myoclonic Seizures in Patients 12 Years of Age and Older with Juvenile Myoclonic Epilepsy
Primary Generalized Tonic-Clonic Seizures
Adults 16 Years and Older
Pediatric Patients Ages 6 to <16 Years
Adult Patients with Impaired Renal Function
HOW SUPPLIED
STORAGE AND HANDLING
Pharmacist
SPL MEDGUIDE
Levetiracetam Tablets, 250 mg, 500 mg and 750 mgWhat is the most important information I should know about levetiracetam tablets?
Like other antiepileptic drugs, levetiracetam tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500 people taking it.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● Stopping levetiracetam tablets suddenly can cause serious problems. Stopping a seizure medicine suddenly can cause seizures that will not stop (status epilepticus).
-
● Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
-
● Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
-
● partial onset seizures in people 4 years of age and older with epilepsy.
-
● myoclonic seizures in people 12 years of age and older with juvenile myoclonic epilepsy
-
● primary generalized tonic-clonic seizures in people 6 years of age and older with certain types of generalized epilepsy.
What should I tell my healthcare provider before starting levetiracetam tablets?
-
● have or have had depression, mood problems or suicidal thoughts or behavior
-
● have kidney problems
-
● are pregnant or planning to become pregnant. It is not known if levetiracetam tablets will harm your unborn baby. You and your healthcare provider will have to decide if you should take levetiracetam tablets while you are pregnant. If you become pregnant while taking levetiracetam tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334.
-
● are breast feeding. Levetiracetam tablets can pass into your milk and may harm your baby. You and your healthcare provider should discuss whether you should take levetiracetam tablets or breast-feed; you should not do both.
How should I take levetiracetam tablets?
-
● Your healthcare provider will tell you how much levetiracetam tablets to take and when to take it . Levetiracetam tablets are usually taken twice a day. Take levetiracetam tablets at the same times each day.
-
● Your healthcare provider may change your dose. Do not change your dose without talking to your healthcare provider.
-
● Take levetiracetam tablets with or without food.
-
● Swallow the tablets whole. Do not chew or crush tablets. Ask your healthcare provider for levetiracetam oral solution if you cannot swallow tablets.
-
● If your healthcare provider has prescribed levetiracetam oral solution, be sure to ask your pharmacist for a medicine dropper or medicine cup to help you measure the correct amount of levetiracetam oral solution. Do not use a household teaspoon. Ask your pharmacist for instructions on how to use the measuring device the right way.
-
● If you miss a dose of levetiracetam tablets, take it as soon as you remember. If it is almost time for your next dose, just skip the missed dose. Take the next dose at your regular time.Do not take two doses at the same time.
-
● If you take too much levetiracetam tablets, call your local Poison Control Center or go to the nearest emergency room right away.
What are the possible side effects of levetiracetam tablets?
-
● SeeWhat is the most important information I should know about levetiracetam tablets?
-
● mood and behavior changes such as aggression, agitation, anger, anxiety, apathy, mood swings, depression, hostility, and irritability. A few people may get psychotic symptoms such as hallucinations (seeing or hearing things that are really not there), delusions (false or strange thoughts or beliefs) and unusual behavior.
-
● extreme sleepiness, tiredness, and weakness
-
● problems with muscle coordination (problems walking and moving)
-
● sleepiness
-
● weakness
-
● dizziness
-
● infection
-
● accidental injury
-
● irritability
-
● hostility
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store levetiracetam tablets?
-
● Store levetiracetam tablets at 20to 25(68to 77to 30(59to 86
-
● Keep levetiracetam tablets and all medicines out of the reach of children.
What are the ingredients of levetiracetam tablets?
Levetiracetam tablet:
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

LevetiracetamLevetiracetam TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!