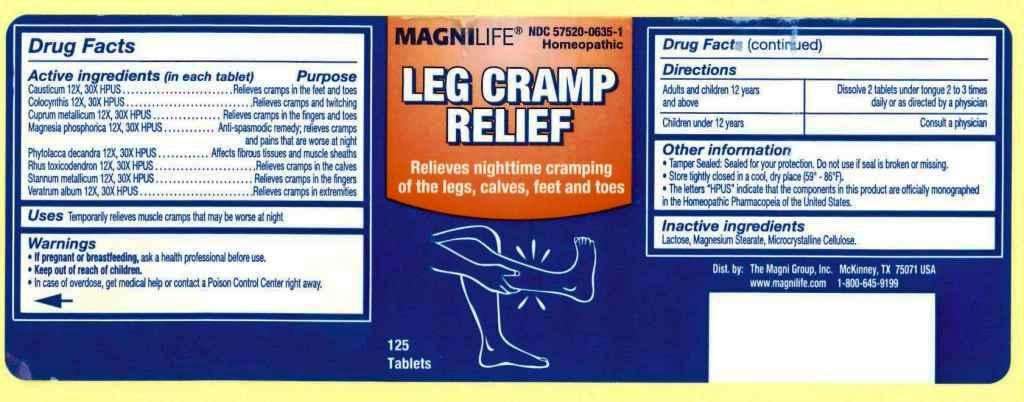
Leg Cramp Relief
Leg Cramp Relief
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENTS: Causticum 12X, 30X, Colocynthis 12X, 30X, Cuprum metallicum 12X, 30X, Magnesia phosphorica 12X, 30X, Phytolacca decandra 12X, 30X, Rhus toxicodendron 12X, 30X, Stannum metallicum 12X, 30X, Veratrum album 12X, 30X.
Purpose
USES: Temporarily relieves muscle cramps that may be worse at night.
WARNINGS: If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
OTHER INFORMATION: Tamper sealed: Sealed for your protection. Do not use if seal is broken or missing.
Store tightly closed in a cool, dry place (59-86 F).
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopeia of the United States.
DIRECTIONS: Adults and children 12 years and above: Dissolve 2 tablets under tongue 2 to 3 times daily or as directed by a physician.
Children under 12 years: Consult a physician.
INACTIVE INGREDIENTS: Lactose, Magnesium stearate, Microcrystalline cellulose.
KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or contact a Poison Control Center right away.
Uses
USES: Temporarily relieves muscle cramps that may be worse at night.
Dist. by:
The Magni Group, Inc.
McKinney, TX 75071 USA
www.magnilife.com
1-800-645-9199
MAGNILIFE
NDC 57520-0635-1
Homeopathic
LEG CRAMP RELIEF
Relieves nighttime cramping of the legs, calves, feet and toes
125 Tablets
Leg Cramp ReliefCausticum, Colocynthis, Cuprum metallicum, Magnesia phosphorica, Phytolacca decandra, Rhus toxicodendron, Stannum metallicum, Veratrum album, TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||