LBel
EFFET PARFAIT
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- LBel Uses
- Warnings
- Directions
- LBel Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 11 g Case
FULL PRESCRIBING INFORMATION
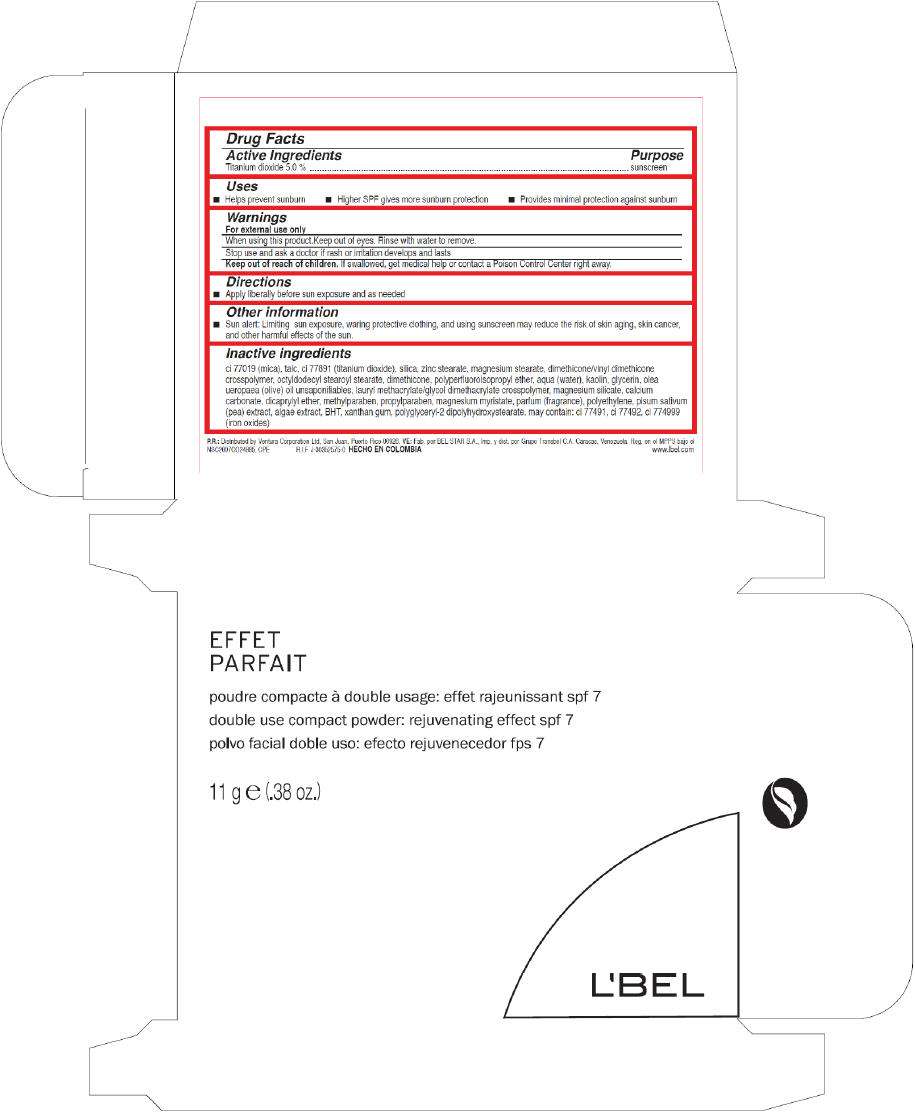
Drug Facts
Active Ingredients
Titanium dioxide 5.0 %
Purpose
sunscreen
LBel Uses
- Helps prevent sunburn
- Higher SPF gives more sunburn protection
- Provides minimal protection against sunburn
Warnings
For external use only.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash or irritation develops and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally before sun exposure and as needed.
LBel Other information
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreen may reduce the risk of skin aging, skin cancer, and other harmful effects of the sun.
Inactive ingredients
talc, mica, zinc stearate, octyldodecyl stearoyl stearate, magnesium stearate, dimethicone/vinyl dimethicone crosspolymer, dimethicone, aqua (water), polyperfluoroisopropyl ether, kaolin, glycerin, olea europaea oil unsaponifiables (olea europaea (olive) oil unsaponifiables), lauryl methacrylate/glycol dimethacrylate crosspolymer, calcium carbonate, magnesium silicate, dicaprylyl ether, methylparaben, propylparaben, magnesium myristate, silica, parfum (fragrance), polyethylene, pisum sativum extract (pisum sativum (pea) extract), algae extract, bht, xanthan gum, polyglyceryl-2 dipolyhydroxystearate. May contain: ci 77891 (titanium dioxide), ci 77492 (iron oxides), ci 77491 (iron oxides), ci 77499 (iron oxides).
PRINCIPAL DISPLAY PANEL - 11 g Case
EFFET
PARFAIT
double use compact powder: rejuvenating effect spf 7
11 g e (.38 oz.)
L'BEL
LBelTitanium dioxide POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||