LBel
L'BEL Effet Parfait Rouge Alissant SPF17
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- LBel Uses
- Warnings
- Directions
- LBel Other information
- Inactive Ingredients
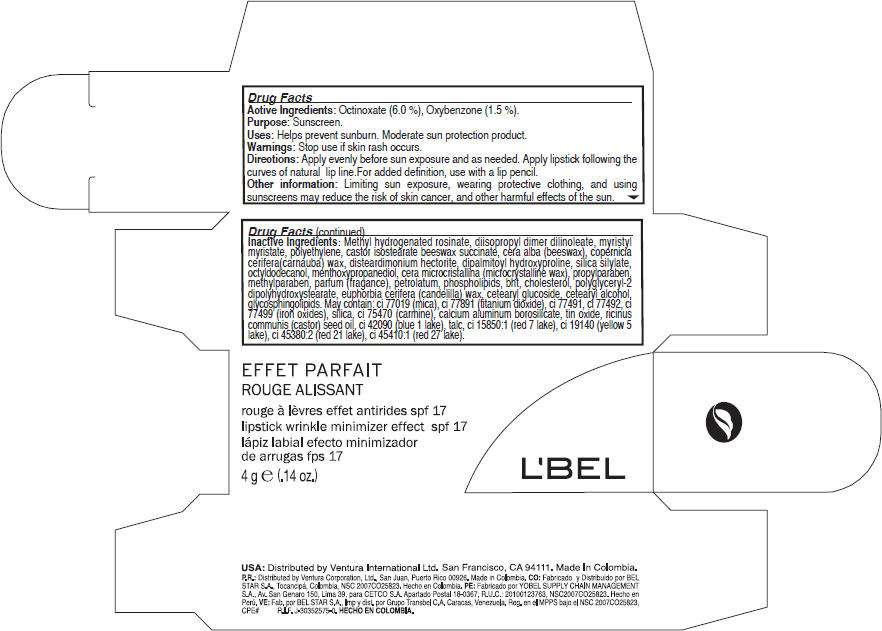
- PRINCIPAL DISPLAY PANEL - 4 g Carton
FULL PRESCRIBING INFORMATION
Active Ingredients
Octinoxate (6.0 %), Oxybenzone (1.5 %).
Purpose
Sunscreen
LBel Uses
Helps prevent sunburn. Moderate sun protection product.
Warnings
Stop use if skin rash occurs.
Directions
Apply evenly before sun exposure and as needed. Apply lipstick following the curves of natural lip line.For added definition, use with a lip pencil.
LBel Other information
Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risk of skin cancer, and other harmful effects of the sun.
Inactive Ingredients
Methyl hydrogenated rosinate, diisopropyl dimer dilinoleate, myristyl myristate,myristyl myristate, castor isostearate beeswax succinate, cera alba (beeswax), copernicia cerifera(carnauba) wax, disteardimonium hectorite, dipalmitoyl hydroxyproline, silica silylate, octyldodecanol, menthoxypropanediol, cera microcristallina (microcrystalline wax), propylparaben, methylparaben, parfum (fragance), petrolatum, phospholipids, bht, cholesterol, polyglyceryl-2 dipolyhydroxystearate, euphorbia cerifera (candelilla) wax, cetearyl glucoside, cetearyl alcohol, glycosphingolipids. May contain: ci 77019 (mica), ci 77891 (titanium dioxide), ci 77491, ci 77492, ci 77499 (iron oxides), silica, ci 75470 (carmine), calcium aluminum borosilicate, tin oxide, ricinus communis (castor) seed oil, ci 42090 (blue 1 lake), talc, ci 15850:1 (red 7 lake), ci 19140 (yellow 5 lake), ci 45380:2 (red 21 lake), ci 45410:1 (red 27 lake).
US: Distributed by Ventura International, Ltd. San Francisco, CA 94111
PRINCIPAL DISPLAY PANEL - 4 g Carton
EFFET PARFAIT
ROUGE ALISSANT
lipstick wrinkle minimizer effect spf 17
4 g e (.14 oz.)
L'BEL
LBelOctinoxate and Oxybenzone LIPSTICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||