Lamotrigine
NorthStar RxLLC
Alembic Pharmaceuticals Limited
HIGHLIGHTS OF PRESCRIBING INFORMATION Rx onlyThese highlights do not include all the information needed to use lamotrigine safely and effectively. See full prescribing information for lamotrigine.Lamotrigine TabletsInitial U.S. Approval: 1994 RECENT MAJOR CHANGESWarnings and Precautions, Aseptic Meningitis (5.7) October 2010BOXED WARNINGWARNING: SERIOUS SKIN RASHES See full prescribing information for complete boxed warning. Cases of life-threatening serious rashes, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and/or rash-related death, have been caused by lamotrigine. The rate of serious rash is greater in pediatric patients than in adults. Additional factors that may increase the risk of rash include ( 5.1 ). coadministration with valproate exceeding recommended initial dose of lamotrigine tablets exceeding recommended dose escalation of lamotrigine tablets Benign rashes are also caused by lamotrigine tablets ; however, it is not possible to predict which rashes will prove to be serious or life-threatening. Lamotrigine tablets should be discontinued at the first sign of rash, unless the rash is clearly not drug-related. (5.1) INDICATIONS AND USAGELamotrigine is an antiepileptic drug (AED) indicated for: Epilepsy - adjunctive therapy in patients ≥2 years of age: (1.1) partial seizures. primary generalized tonic-clonic seizures. generalized seizures of Lennox-Gastaut syndrome. Epilepsy - monotherapy in patients ≥16 years of age: conversion to monotherapy in patients with partial seizures who are receiving treatment with carbamazepine, phenobarbital, phenytoin, primidone, or valproate as the single AED. (1.1) Bipolar Disorder in patients ≥18 years of age: maintenance treatment of Bipolar I Disorder to delay the time to occurrence of mood episodes in patients treated for acute mood episodes with standard therapy. (1.2)DOSAGE AND ADMINISTRATION Dosing is based on concomitant medications, indication, and patient age. (2.2, 2.4) To avoid an increased risk of rash, the recommended initial dose and subsequent dose escalations should not be exceeded. Do not restart lamotrigine tablets in patients who discontinued due to rash unless the potential benefits clearly outweigh the risks. (2.1) Adjustments to maintenance doses will in most cases be required in patients starting or stopping estrogen-containing oral contraceptives. (2.1, 5.9) Lamotrigine tablets should be discontinued over a period of at least 2 weeks (approximately 50% reduction per week). (2.1, 5.10) Epilepsy Adjunctive therapy - See Table 1 for patients >12 years of age and Tables 2 and 3 for patients 2 to 12 years. (2.2) Conversion to monotherapy - See Table 4. (2.3) Bipolar Disorder: See Tables 5 and 6 (2.4) DOSAGE FORMS AND STRENGTHS Tablets: 25 mg, 100 mg, 150 mg, and 200 mg scored. (3.1, 16)CONTRAINDICATIONS Hypersensitivity to the drug or its ingredients. (Boxed Warning, 4) WARNINGS AND PRECAUTIONS Life-threatening serious rash and/or rash-related death may result. (Boxed Warning, 5.1) Hypersensitivity reaction may be fatal or life-threatening. Early signs of hypersensitivity (e.g., fever, lymphadenopathy) may present without rash; if signs present, patient should be evaluated immediately. Lamotrigine tablets should be discontinued if alternate etiology for hypersensitivity signs is not found. (5.2) Acute multiorgan failure has resulted (some cases fatal). (5.3) Blood dyscrasias (e.g., neutropenia, thrombocytopenia, pancytopenia), may result either with or without an associated hypersensitivity syndrome. (5.4) Suicidal behavior and ideation. (5.5) Clinical worsening, emergence of new symptoms, and suicidal ideation / behaviors may be associated with treatment of bipolar disorder. Patients should be closely monitored, particularly early in treatment or during dosage changes. (5.6) Aseptic meningitis reported in pediatric and adult patients. (5.7) Medication errors involving lamotrigine tablets have occurred. In particular the names Lamotrigine can be confused with names of other commonly used medications. Medication errors may also occur between the different formulations of lamotrigine tablets (3.4, 5.8, 16, 17.9) Side Effects Most common adverse reactions (incidence ≥10%) in adult epilepsy clinical studies were dizziness, headache, diplopia, ataxia, nausea, blurred vision, somnolence, rhinitis, and rash. Additional adverse reactions (incidence ≥10%) reported in children in epilepsy clinical studies included vomiting, infection, fever, accidental injury, pharyngitis, abdominal pain, and tremor. (6.1) Most common adverse reactions (incidence >5%) in adult bipolar clinical studies were nausea, insomnia, somnolence, back pain, fatigue, rash, rhinitis, abdominal pain, and xerostomia. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS Valproate increases lamotrigine concentrations more than 2-fold. (7, 12.3) Carbamazepine, phenytoin, phenobarbital, and primidone decrease lamotrigine concentrations by approximately 40%. (7, 12.3) Oral estrogen-containing contraceptives and rifampin also decrease lamotrigine concentrations by approximately 50%. (7, 12.3) USE IN SPECIFIC POPULATIONS Hepatic impairment: Dosage adjustments required. (2.1) Healthcare professionals can enroll patients in the Lamotrigine Pregnancy Registry (1-800-336-2176). Patients can enroll themselves in the North American Antiepileptic Drug Pregnancy Registry (1-888-233-2334). (8.1) Efficacy of lamotrigine tablets, used as adjunctive treatment for partial seizures, was not demonstrated in a small randomized, double-blind, placebo-controlled study in very young pediatric patients (1 to 24 months). (8.4)
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: SERIOUS SKIN RASHES
- 1. LAMOTRIGINE INDICATIONS AND USAGE
- 2. LAMOTRIGINE DOSAGE AND ADMINISTRATION
- 3. DOSAGE FORMS AND STRENGTHS
- 4. LAMOTRIGINE CONTRAINDICATIONS
- 5. WARNINGS AND PRECAUTIONS
- 5.1. Serious Skin Rashes [see Boxed Warning]
- 5.2. Hypersensitivity Reactions
- 5.3. Acute Multiorgan Failure
- 5.4. Blood Dyscrasias
- 5.5. Suicidal Behavior and Ideation
- 5.6. Use in Patients With Bipolar Disorder
- 5.7. Aseptic Meningitis
- 5.8. Potential Medication Errors
- 5.9. Concomitant Use With Oral Contraceptives
- 5.10. Withdrawal Seizures
- 5.11. Status Epilepticus
- 5.12. Sudden Unexplained Death in Epilepsy (SUDEP)
- 5.13. Addition of Lamotrigine Tablets to a Multidrug Regimen That Includes Valproate
- 5.14. Binding in the Eye and Other Melanin-Containing Tissues
- 5.15. Laboratory Tests
- 6. LAMOTRIGINE ADVERSE REACTIONS
- 7. DRUG INTERACTIONS
- 8. USE IN SPECIFIC POPULATIONS
- 10. OVERDOSAGE
- 11. LAMOTRIGINE DESCRIPTION
- 12. CLINICAL PHARMACOLOGY
- 13. NONCLINICAL TOXICOLOGY
- 14. CLINICAL STUDIES
- 16. HOW SUPPLIED/STORAGE AND HANDLING
- 17. PATIENT COUNSELING INFORMATION
- MEDICATION GUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
WARNING: SERIOUS SKIN RASHES
Lamotrigine can cause serious rashes requiring hospitalization and discontinuation of treatment. The incidence of these rashes, which have included Stevens-Johnson syndrome, is approximately 0.8% (8 per 1,000) in pediatric patients (2 to 16 years of age) receiving lamotrigine tablets as adjunctive therapy for epilepsy and 0.3% (3 per 1,000) in adults on adjunctive therapy for epilepsy. In clinical trials of bipolar and other mood disorders, the rate of serious rash was 0.08% (0.8 per 1,000) in adult patients receiving lamotrigine tablets as initial monotherapy and 0.13% (1.3 per 1,000) in adult patients receiving lamotrigine tablets as adjunctive therapy. In a prospectively followed cohort of 1,983 pediatric patients (2 to 16 years of age) with epilepsy taking adjunctive lamotrigine tablets, there was 1 rash-related death. In worldwide postmarketing experience, rare cases of toxic epidermal necrolysis and/or rash-related death have been reported in adult and pediatric patients, but their numbers are too few to permit a precise estimate of the rate.
Other than age, there are as yet no factors identified that are known to predict the risk of occurrence or the severity of rash caused by lamotrigine tablets . There are suggestions, yet to be proven, that the risk of rash may also be increased by (1) coadministration of lamotrigine tablets with valproate (includes valproic acid and divalproex sodium), (2) exceeding the recommended initial dose of lamotrigine tablets, or (3) exceeding the recommended dose escalation for lamotrigine tablet. However, cases have occurred in the absence of these factors.
Nearly all cases of life-threatening rashes caused by lamotrigine tablets have occurred within 2 to 8 weeks of treatment initiation. However, isolated cases have occurred after prolonged treatment (e.g., 6 months). Accordingly, duration of therapy cannot be relied upon as means to predict the potential risk heralded by the first appearance of a rash.
Although benign rashes are also caused by lamotrigine tablets, it is not possible to predict reliably which rashes will prove to be serious or life-threatening. Accordingly, lamotrigine tablets should ordinarily be discontinued at the first sign of rash, unless the rash is clearly not drug-related. Discontinuation of treatment may not prevent a rash from becoming life-threatening or permanently disabling or disfiguring [see Warnings and Precautions (5.1)].
1. INDICATIONS AND USAGE
1.1. Epilepsy
Adjunctive Therapy: Lamotrigine tablets are indicated as adjunctive therapy for the following seizure types in patients ≥2 years of age:
- partial seizures
- primary generalized tonic-clonic seizures.
- generalized seizures of Lennox-Gastaut syndrome
Monotherapy: Lamotrigine tablets are indicated for conversion to monotherapy in adults (≥16 years of age) with partial seizures who are receiving treatment with carbamazepine, phenytoin, phenobarbital, primidone, or valproate as the single antiepileptic drug (AED).
Safety and effectiveness of lamotrigine tablets have not been established (1) as initial monotherapy; (2) for conversion to monotherapy from AEDs other than carbamazepine, phenytoin, phenobarbital, primidone, or valproate; or (3) for simultaneous conversion to monotherapy from 2 or more concomitant AEDs.
1.2. Bipolar Disorder
Lamotrigine tablets are indicated for the maintenance treatment of Bipolar I Disorder to delay the time to occurrence of mood episodes (depression, mania, hypomania, mixed episodes) in adults (≥18 years of age) treated for acute mood episodes with standard therapy. The effectiveness of lamotrigine tablets in the acute treatment of mood episodes has not been established.
The effectiveness of lamotrigine tablets as maintenance treatment was established in 2 placebo-controlled trials in patients with Bipolar I Disorder as defined by DSM-IV [see Clinical Studies (14.2)]. The physician who elects to prescribe lamotrigine tablets for periods extending beyond 16 weeks should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
2. DOSAGE AND ADMINISTRATION
2.1. General Dosing Considerations
Rash[see Boxed Warning]
[see Clinical Pharmacology (12.3)].
Lamotrigine tablets Added to Drugs Known to Induce or Inhibit Glucuronidation[see Clinical Pharmacology (12.3)]
Target Plasma Levels for Patients With Epilepsy or Bipolar Disorder[see Clinical Pharmacology (12.3)]
Women Taking Estrogen-Containing Oral ContraceptivesStarting Lamotrigine Tablets in Women Taking Estrogen-Containing Oral Contraceptives: [see Clinical Pharmacology (12.3)]
Adjustments to the Maintenance Dose of Lamotrigine Tablets In Women Taking Estrogen-Containing Oral Contraceptives:
- Taking Estrogen-Containing Oral Contraceptives: For women not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin that induce lamotrigine glucuronidation [see Drug Interactions (7), Clinical Pharmacology (12.3)], the maintenance dose of lamotrigine tablets will in most cases need to be increased, by as much as 2-fold over the recommended target maintenance dose, in order to maintain a consistent lamotrigine plasma level [see Clinical Pharmacology (12.3)].
- Starting Estrogen-Containing Oral Contraceptives: In women taking a stable dose of lamotrigine tablets and not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin that induce lamotrigine glucuronidation [see Drug Interactions (7), Clinical Pharmacology (12.3)], the maintenance dose will in most cases need to be increased by as much as 2-fold, in order to maintain a consistent lamotrigine plasma level. The dose increases should begin at the same time that the oral contraceptive is introduced and continue, based on clinical response, no more rapidly than 50 to 100 mg/day every week. Dose increases should not exceed the recommended rate (see Table 1 or Table 5) unless lamotrigine plasma levels or clinical response support larger increases. Gradual transient increases in lamotrigine plasma levels may occur during the week of inactive hormonal preparation (“pill-free” week), and these increases will be greater if dose increases are made in the days before or during the week of inactive hormonal preparation. Increased lamotrigine plasma levels could result in additional adverse reactions, such as dizziness, ataxia, and diplopia. If adverse reactions attributable to lamotrigine tablets consistently occur during the “pill-free” week, dose adjustments to the overall maintenance dose may be necessary. Dose adjustments limited to the “pill-free” week are not recommended. For women taking lamotrigine tablets in addition to carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin that induce lamotrigine glucuronidation [see Drug Interactions (7), Clinical Pharmacology (12.3)], no adjustment to the dose of lamotrigine should be necessary.
- Stopping Estrogen-Containing Oral Contraceptives: For women not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin that induce lamotrigine glucuronidation [see Drug Interactions (7), Clinical Pharmacology (12.3)], the maintenance dose of lamotrigine tablets will in most cases need to be decreased by as much as 50%, in order to maintain a consistent lamotrigine plasma level. The decrease in dose of lamotrigine tablets should not exceed 25% of the total daily dose per week over a 2-week period, unless clinical response or lamotrigine plasma levels indicate otherwise [see Clinical Pharmacology (12.3)]. For women taking lamotrigine tablets in addition to carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin that induce lamotrigine glucuronidation [see Drug Interactions (7), Clinical Pharmacology (12.3)], no adjustment to the dose of lamotrigine tablets should be necessary.
Women and Other Hormonal Contraceptive Preparations or Hormone Replacement Therapy:
Patients With Hepatic Impairment: [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)],
Patients With Renal Impairment [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)]
Discontinuation Strategy: Epilepsy:
[see Warnings and Precautions (5.10)].
Bipolar Disorder: [see Warnings and Precautions (5.10)]
2.2. Epilepsy - Adjunctive Therapy
Patients Over 12 Years of Age:
Table 1
Escalation Regimen for Lamotrigine Tablets in Patients Over 12 Years of Age With Epilepsy
| |
For Patients TAKING Valproatea
|
For Patients NOT TAKING Carbamazepine, Phenytoin, Phenobarbital, Primidoneb, or Valproatea
|
For Patients TAKING Carbamazepine, Phenytoin, Phenobarbital, or Primidoneb and NOT TAKING Valproatea
|
| Weeks 1 and 2 |
25 mg every
other
day
|
25 mg every day
|
50 mg/day
|
| Weeks 3 and 4 |
25 mg every day
|
50 mg/day
|
100 mg/day
(in 2 divided doses) |
| Week 5 onwards to maintenance |
Increase by 25 to 50 mg/day every 1 to 2 weeks |
Increase by 50 mg/day every 1 to 2 weeks |
Increase by 100 mg/day every 1 to 2 weeks. |
| Usual Maintenance Dose |
100 to 200 mg/day with valproate alone
100 to 400 mg/day with valproate and other drugs that induce glucuronication ( in 1 or 2 divided doses) |
225 to 375 mg/day
(in 2 divided doses). |
300 to 500 mg/day
(in 2 divided doses). |
a [see Drug Interactions (7), Clinical Pharmacology (12.3)].
b [see Drug Interactions (7) Clinical Pharmacology (12.3)][see Drug Interactions (7), Clinical Pharmacology (12.3)]. [see Dosage and Administration (2.1)].
Patients 2 to 12 Years of Age:
Smaller starting doses and slower dose escalations than those used in clinical trials are recommended because of the suggestions that the risk of rash may be decreased by smaller starting doses and slower dose escalations. Therefore, maintenance doses will take longer to reach in clinical practice than in clinical trials. It may take several weeks to months to achieve an individualized maintenance dose. Maintenance doses in patients weighing less than 30 kg, regardless of age or concomitant AED, may need to be increased as much as 50%, based on clinical response.
Table 2
Escalation Regimen for Lamotrigine Tablets in Patients 2 to 12 Years of Age With Epilepsy
| |
For Patients TAKING Valproatea
|
For Patients NOT TAKING Carbamazepine, Phenytoin, Phenobarbital, Primidoneb, or Valproatea |
For Patients TAKING Carbamazepine, Phenytoin, Phenobarbital, or Primidoneb and NOT TAKING Valproatea
|
| Weeks 1 and 2 |
0.15 mg/kg/day
in 1 or 2 divided doses, rounded down to the nearest whole tablet (see Table 3 for weight based dosing guide). |
0.3 mg/kg/day
in 1 or 2 divided doses, rounded down to the nearest whole tablet. |
0.6 mg/kg/day
in 2 divided doses, rounded down to the nearest whole tablet. |
| Weeks 3 and 4 |
0.3 mg/kg/day
in 1 or 2 divided doses, rounded down to the nearest whole tablet (see Table 3 for weight based dosing guide). |
0.6 mg/kg/day
in 2 divided doses, rounded down to the nearest whole tablet. |
1.2 mg/kg/day
in 2 divided doses, rounded down to the nearest whole tablet. |
| Week 5 onwards to maintenance |
The dose should be increased every 1 to 2 weeks as follows: calculate 0.3 mg/kg/day, round this amount down to the nearest whole tablet, and add this amount to the previously administered daily dose. |
The dose should be increased every 1 to 2 weeks as follows: calculate 0.6 mg/kg/day, round this amount down to the nearest whole tablet, and add this amount to the previously administered daily dose |
The dose should be increased every 1 to 2 weeks as follows: calculate 1.2 mg/kg/day, round this amount down to the nearest whole tablet, and add this amount to the previously administered daily dose |
| Usual Maintenance Dose |
1 to 5 mg/kg/day(maximum 200 mg/day in 1 or 2 divided doses). 1 to 3 mg/kg/day with valproate alone |
4.5 to 7.5 mg/kg/day
(maximum 300 mg/day in 2 divided doses) |
5 to 15 mg/kg/day
(maximum 400 mg/day in 2 divided doses) |
| Maintenance dose in patients less than 30 kg |
May need to be increased by as much as 50%, based on clinical response |
May need to be increased by as much as 50%, based on clinical response |
May need to be increased by as much as 50%, based on clinical response |
Note: Only whole tablets should be used for dosing
a [see Drug Interactions (7), Clinical Pharmacology (12.3)].
b [see Drug Interactions (7) Clinical Pharmacology (12.3)][see Drug Interactions (7), Clinical Pharmacology (12.3)]. [see Dosage and Administration (2.1)].
Table 3
The Initial Weight-Based Dosing Guide for Patients 2 to 12 Years Taking Valproate (Weeks 1 to 4) With Epilepsy
| If the patient’s weight is |
Give this daily dose, using the most appropriate combination of lamotrigine tablets 2-mg and 5-mg tablets |
||
| Greater than |
And less than |
Weeks 1 and 2 |
Weeks 3 and 4 |
| 6.7 kg |
14 kg |
2 mg every other day |
2 mg every day |
| 14.1 kg |
27 kg |
2 mg every day |
4 mg every day |
| 27.1 kg |
34 kg |
4 mg every day |
8 mg every day |
| 34.1 kg |
40 kg |
5 mg every day |
10 mg every day |
Usual Maintenance Dose for Epilepsy:without valproatevalproate alone
2.3. Epilepsy - Conversion From Adjunctive Therapy to Monotherapy
The goal of the transition regimen is to effect the conversion to monotherapy with lamotrigine tablet under conditions that ensure adequate seizure control while mitigating the risk of serious rash associated with the rapid titration of lamotrigine tablet.
The recommended maintenance dose of lamotrigine tablet as monotherapy is 500 mg/day given in 2 divided doses.
[see Boxed Warning]
Conversion From Adjunctive Therapy With Carbamazepine, Phenytoin, Phenobarbital, or Primidone to Monotherapy with Lamotrigine Tablets:
Conversion from Adjunctive Therapy with Valproate to Monotherapy with Lamotrigine Tablets:
Table 4
Conversion From Adjunctive Therapy with Valproate to Monotherapy with Lamotrigine Tablets in Patients ≥16 Years of Age with Epilepsy
| |
Lamotrigine Tablets |
Valproate |
| Step 1 |
Achieve a dose of 200 mg/day according to guidelines in Table 1 (if not already on 200 mg/day). |
Maintain previous stable dose. |
| Step 2 |
Maintain at 200 mg/day. |
Decrease to 500 mg/day by decrements no greater than 500 mg/day per week and then maintain the dose of 500 mg/day for 1 week. |
| Step 3 |
Increase to 300 mg/day and maintain for 1 week. |
Simultaneously decrease to 250 mg/day and maintain for 1 week. |
| Step 4 |
Increase by 100 mg/day every week to achieve maintenance dose of 500 mg/day. |
Discontinue. |
Conversion from Adjunctive Therapy With AEDs Other Than Carbamazepine, Phenytoin, Phenobarbital, Primidone, or Valproate to Monotherapy With Lamotrigine Tablets:
2.4. Bipolar Disorder
see Clinical Studies (14.2)
[see Drug Interactions (7), Clinical Pharmacology (12.3)]
[see Boxed Warning]
Table 5
Escalation Regimen for Lamotrigine Tablets for Patients with Bipolar Disorder
| |
For Patients TAKING Valproatea
|
For Patients NOT TAKING Carbamazepine, Phenytoin, Phenobarbital, Primidoneb, or Valproatea
|
For Patients TAKING Carbamazepine, Phenytoin, Phenobarbital, or Primidoneb and NOT TAKING Valproatea
|
| Weeks 1 and 2 |
25 mg every other day |
25 mg daily |
50 mg daily |
| Weeks 3 and 4 |
25 mg daily |
50 mg daily |
100 mg daily, in divided doses |
| Week 5 |
50 mg daily |
100 mg daily |
200 mg daily, in divided doses |
| Week 6 |
100 mg daily |
200 mg daily |
300 mg daily, in divided doses |
| Week 7 |
100 mg daily |
200 mg daily |
up to 400 mg daily, in divided doses |
a [see Drug Interactions (7), Clinical Pharmacology (12.3)]
b [see Drug Interactions (7), Clinical Pharmacology (12.3)][see Drug Interactions (7), Clinical Pharmacology (12.3)][see Dosage and Administration (2.1)]
Table 6
Dosage Adjustments to Lamotrigine Tablets for Patients with Bipolar Disorder Following Discontinuation of Psychotropic Medications
| |
Discontinuation of Psychotropic Drugs (excluding Carbamazepine, Phenytoin, Phenobarbital, Primidoneb, or Valproatea) |
After Discontinuation of Valproatea
|
After Discontinuation of Carbamazepine, Phenytoin, Phenobarbital, or Primidoneb
|
| Current dose of Lamotrigine Tablets (mg/day) 100 |
Current dose of Lamotrigine Tablets (mg/day) 400 |
||
| Week 1 |
Maintain current dose of Lamotrigine Tablets |
150 |
400 |
| Week 2 |
Maintain current dose of Lamotrigine Tablets |
200 |
300 |
| Week 3 onward |
Maintain current dose of Lamotrigine Tablets |
200 |
200 |
a [see Drug Interactions (7), Clinical Pharmacology (12.3)]
b [see Drug Interactions (7), Clinical Pharmacology (12.3)][see Drug Interactions (7), Clinical Pharmacology (12.3)][see Dosage and Administration (2.1)]
[see Clinical Studies (14.2)]
3. DOSAGE FORMS AND STRENGTHS
3.1. Tablets
capsule shaped
round
round
round
3.4. Potential Medication Errors
.
4. CONTRAINDICATIONS
[see Boxed Warning, Warnings and Precautions (5.1), (5.2)]
5. WARNINGS AND PRECAUTIONS
5.1. Serious Skin Rashes [see Boxed Warning]
Pediatric Population: The incidence of serious rash associated with hospitalization and discontinuation of lamotrigine tablets in a prospectively followed cohort of pediatric patients (2 to 16 years of age) with epilepsy receiving adjunctive therapy was approximately 0.8% (16 of 1,983). When 14 of these cases were reviewed by 3 expert dermatologists, there was considerable disagreement as to their proper classification. To illustrate, one dermatologist considered none of the cases to be Stevens-Johnson syndrome; another assigned 7 of the 14 to this diagnosis. There was 1 rash-related death in this 1,983-patient cohort. Additionally, there have been rare cases of toxic epidermal necrolysis with and without permanent sequelae and/or death in US and foreign postmarketing experience.
There is evidence that the inclusion of valproate in a multidrug regimen increases the risk of serious, potentially life-threatening rash in pediatric patients. In pediatric patients who used valproate concomitantly, 1.2% (6 of 482) experienced a serious rash compared to 0.6% (6 of 952) patients not taking valproate.
Adult Population: Serious rash associated with hospitalization and discontinuation of lamotrigine tablets occurred in 0.3% (11 of 3,348) of adult patients who received lamotrigine tablets in premarketing clinical trials of epilepsy. In the bipolar and other mood disorders clinical trials, the rate of serious rash was 0.08% (1 of 1,233) of adult patients who received lamotrigine tablets as initial monotherapy and 0.13% (2 of 1,538) of adult patients who received lamotrigine tablets as adjunctive therapy. No fatalities occurred among these individuals. However, in worldwide postmarketing experience, rare cases of rash-related death have been reported, but their numbers are too few to permit a precise estimate of the rate.
Among the rashes leading to hospitalization were Stevens-Johnson syndrome, toxic epidermal necrolysis, angioedema, and a rash associated with a variable number of the following systemic manifestations: fever, lymphadenopathy, facial swelling, hematologic, and hepatologic abnormalities.
There is evidence that the inclusion of valproate in a multidrug regimen increases the risk of serious, potentially life-threatening rash in adults. Specifically, of 584 patients administered lamotrigine tablets with valproate in epilepsy clinical trials, 6 (1%) were hospitalized in association with rash; in contrast, 4 (0.16%) of 2,398 clinical trial patients and volunteers administered lamotrigine tablets in the absence of valproate were hospitalized.
Patients With History of Allergy or Rash to Other AEDs: The risk of nonserious rash may be increased when the recommended initial dose and/or the rate of dose escalation of lamotrigine tablets is exceeded and in patients with a history of allergy or rash to other AEDs.
5.2. Hypersensitivity Reactions
Prior to initiation of treatment with lamotrigine tablets, the patient should be instructed that a rash or other signs or symptoms of hypersensitivity (e.g., fever, lymphadenopathy) may herald a serious medical event and that the patient should report any such occurrence to a physician immediately.
5.3. Acute Multiorgan Failure
5.4. Blood Dyscrasias
5.5. Suicidal Behavior and Ideation
Table 7 Risk by Indication for Antiepileptic Drugs in the Pooled Analysis
| Indication | Placebo Patients With Events Per 1,000 Patients |
Drug Patients With Events Per 1,000 Patients |
Relative Risk: Incidence of Events in Drug Patients / Incidence in Placebo Patients |
Risk Difference: Additional Drug Patients With Events Per 1,000 Patients |
| Epilepsy | 1.0 |
3.4 |
3.5 |
2.4 |
| Psychiatric | 5.7 |
8.5 |
1.5 |
2.9 |
| Other | 1.0 |
1.8 |
1.9 |
0.9 |
| Total | 2.4 |
4.3 |
1.8 |
1.9 |
5.6. Use in Patients With Bipolar Disorder
Acute Treatment of Mood Episodes:
Children and Adolescents (less than 18 years of age):Suicidal Behavior and Ideation (5.5)
Clinical Worsening and Suicide Risk Associated With Bipolar Disorder:
Suicidal Behavior and Ideation (5.5)
see Overdosage (10.1)
5.7. Aseptic Meningitis
[see Warnings and Precautions (5.2)]
5.8. Potential Medication Errors
5.9. Concomitant Use With Oral Contraceptives
[see Clinical Pharmacology (12.3)]Dosage adjustments will be necessary in most patients who start or stop estrogen-containing oral contraceptives while taking lamotrigine tablets [see Dosage and Administration (2.1)]
5.10. Withdrawal Seizures
[see Dosage and Administration (2.1)]
5.11. Status Epilepticus
5.12. Sudden Unexplained Death in Epilepsy (SUDEP)
5.13. Addition of Lamotrigine Tablets to a Multidrug Regimen That Includes Valproate
5.14. Binding in the Eye and Other Melanin-Containing Tissues
[see Clinical Pharmacology (12.2)]
5.15. Laboratory Tests
6. ADVERSE REACTIONS
Warnings and Precautions
- Serious skin rashes [see Warnings and Precautions (5.1)]
- Hypersensitivity reactions [see Warnings and Precautions (5.2)]
- Acute multiorgan failure [see Warnings and Precautions (5.3)]
- Blood dyscrasias [see Warnings and Precautions (5.4)]
- Suicidal behavior and ideation [see Warnings and Precautions (5.5)]
- Aseptic meningitis [see Warnings and Precautions (5.7) ]
- Withdrawal seizures [see Warnings and Precautions (5.10)]
- Status epilepticus [see Warnings and Precautions (5.11)]
- Sudden unexplained death in epilepsy [see Warnings and Precautions (5.12)]
6.1. Clinical Trials
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Lamotrigine tablets have been evaluated for safety in patients with epilepsy and in patients with Bipolar I Disorder. Adverse reactions reported for each of these patient populations are provided below. Excluded are adverse reactions considered too general to be informative and those not reasonably attributable to the use of the drug.
Epilepsy: Most Common Adverse Reactions in All Clinical Studies: Adjunctive Therapy in Adults with Epilepsy:[see Warnings and Precautions (5.1)]
Monotherapy in Adults With Epilepsy:
djunctive Therapy in Pediatric Patients with Epilepsy:
In 339 patients 2 to 16 years of age with partial seizures or generalized seizures of Lennox-Gastaut syndrome, 4.2% of patients on lamotrigine tablets and 2.9% of patients on placebo discontinued due to adverse reactions. The most commonly reported adverse reaction that led to discontinuation of lamotrigine tablets was rash.
Controlled Adjunctive Clinical Studies in Adults With Epilepsy:
Table 8
Treatment-Emergent Adverse Reaction Incidence in Placebo-Controlled Adjunctive Trials in Adult Patients With Epilepsy a (Adverse reactions in at least 2% of patients treated with lamotrigine tablets and numerically more frequent than in the placebo group.)
| Body System/ Adverse Reaction |
Percent of Patients Receiving Adjunctive Lamotrigine Tablets (n = 711) |
Percent of Patients Receiving Adjunctive Placebo (n = 419) |
| Body as a whole Headache Flu syndrome Fever Abdominal pain Neck pain Reaction aggravated (seizure exacerbation) |
29 7 6 5 2 2 |
19 6 4 4 1 1 |
| Digestive Nausea Vomiting Diarrhea Dyspepsia Constipation Anorexia |
19 9 6 5 4 2 |
10 4 4 2 3 1 |
| Musculoskeletal Arthralgia |
2 |
0 |
| Nervous Dizziness Ataxia Somnolence Incoordination Insomnia Tremor Depression Anxiety Convulsion Irritability Speech disorder Concentration disturbance |
38 22 14 6 6 4 4 4 3 3 3 2 |
13 6 7 2 2 1 3 3 1 2 0 1 |
| Respiratory Rhinitis Pharyngitis Cough increased |
14 10 8 |
9 9 6 |
| Skin and appendages Rash Pruritus |
10 3 |
5 2 |
| Special senses Diplopia Blurred vision Vision abnormality |
28 16 3 |
7 5 1 |
| Urogenital Female patients only Dysmenorrhea Vaginitis Amenorrhea |
(n = 365) 7 4 2 |
(n = 207) 6 1 1 |
Table 9
Dose-Related Adverse Reactions From a Randomized, Placebo-Controlled Adjunctive Trial in Adults With Epilepsy
| Adverse Reaction |
Percent of Patients Experiencing Adverse Reactions |
||
| Placebo (n = 73) |
Lamotrigine Tablets 300 mg (n = 71) |
Lamotrigine Tablets 500 mg (n = 72) |
|
| Ataxia Blurred vision Diplopia Dizziness Nausea Vomiting |
10 10 8 27 11 4 |
10 11 24a 31 18 11 |
28ab
25ab 49ab 54ab 25a 18a |
a
b
Controlled Monotherapy Trial in Adults with Partial Seizures:
Table 10
Treatment-Emergent Adverse Reaction Incidence in Adults With Partial Seizures in a Controlled Monotherapy Trial a (Adverse reactions in at least 5% of patients treated with lamotrigine tablets and numerically more frequent than in the valproate group.)
| Body System/ Adverse Reaction |
Percent of Patients Receiving Lamotrigine Tablets as Monotherapy b
(n = 43) |
Percent of Patients Receiving Low-Dose Valproate cMonotherapy (n = 44) |
| Body as a whole Pain Infection Chest pain |
5 5 5 |
0 2 2 |
| Digestive Vomiting Dyspepsia Nausea |
9 7 7 |
0 2 2 |
| Metabolic and nutritional Weight decrease |
5 |
2 |
| Nervous Coordination abnormality Dizziness Anxiety Insomnia |
7 7 5 5 |
0 0 0 2 |
| Respiratory Rhinitis |
7 |
2 |
| Urogenital (female patients only) Dysmenorrhea |
(n = 21) 5 |
(n = 28) 0 |
a
b
c
Body as a Whole:
Digestive:
Metabolic and Nutritional:
Nervous System:
Respiratory:
Skin and Appendages:
Special Senses:
Incidence in Controlled Adjunctive Trials in Pediatric Patients with Epilepsy:
Table 11 lists adverse reactions that occurred in at least 2% of 339 pediatric patients with partial seizures or generalized seizures of Lennox-Gastaut syndrome, who received lamotrigine tablets up to 15 mg/kg/day or a maximum of 750 mg per day. Reported adverse reactions were classified using COSTART terminology.
Table 11
Treatment-Emergent Adverse Reaction Incidence in Placebo-Controlled Adjunctive Trials in Pediatric Patients With Epilepsy (Adverse reactions in at least 2% of patients treated with lamotrigine tablets and numerically more frequent than in the placebo group.)
| Body System/ Adverse Reaction |
Percent of Patients Receiving Lamotrigine Tablets (n = 168) |
Percent of Patients Receiving Placebo (n = 171) |
| Body as a whole Infection Fever Accidental injury Abdominal pain Asthenia Flu syndrome Pain Facial edema Photosensitivity |
20 15 14 10 8 7 5 2 2 |
17 14 12 5 4 6 4 1 0 |
| Cardiovascular Hemorrhage |
2 |
1 |
| Digestive Vomiting Diarrhea Nausea Constipation Dyspepsia |
20 11 10 4 2 |
16 9 2 2 1 |
| Hemic and lymphatic Lymphadenopathy |
2 |
1 |
| Metabolic and nutritional Edema |
2 |
0 |
| Nervous system Somnolence Dizziness Ataxia Tremor Emotional lability Gait abnormality Thinking abnormality Convulsions Nervousness Vertigo |
17 14 11 10 4 4 3 2 2 2 |
15 4 3 1 2 2 2 1 1 1 |
| Respiratory Pharyngitis Bronchitis Increased cough Sinusitis Bronchospasm |
14 7 7 2 2 |
11 5 6 1 1 |
| Skin Rash Eczema Pruritus |
14 2 2 |
12 1 1 |
| Special senses Diplopia Blurred vision Visual abnormality |
5 4 2 |
1 1 0 |
| Urogenital Male and female patients Urinary tract infection |
3 |
0 |
Table 12
Treatment-Emergent Adverse Reaction Incidence in 2 Placebo-Controlled Trials in Adults With Bipolar I Disorder a (Adverse reactions in at least 5% of patients treated with lamotrigine tablets as monotherapy and numerically more frequent than in the placebo group.)
| Body System/ Adverse Reaction |
Percent of Patients Receiving Lamotrigine Tablets n = 227 |
Percent of Patients Receiving Placebo n = 190 |
| General Back pain Fatigue Abdominal pain |
8 8 6 |
6 5 3 |
| Digestive Nausea Constipation Vomiting |
14 5 5 |
11 2 2 |
| Nervous System Insomnia Somnolence Xerostomia (dry mouth) |
10 9 6 |
6 7 4 |
| Respiratory Rhinitis Exacerbation of cough Pharyngitis |
7 5 5 |
4 3 4 |
| Skin Rash (nonserious) b |
7 |
5 |
a
b[see Warnings and Precautions (5.1)].
General:
Cardiovascular:
Digestive:
Metabolic and Nutritional:
Musculoskeletal:
Nervous System:
Respiratory:
Urogenital:
Adverse Reactions Following Abrupt Discontinuation: [see Warnings and Precautions (5.10)]
Mania/Hypomania/Mixed Episodes:
6.2. Other Adverse Side Effects Observed in All Clinical Trials
frequentinfrequent rare
Body as a Whole: Infrequent:
Cardiovascular System: Infrequent:
Dermatological: Infrequent: Rare:
Digestive System: Infrequent: Rare:
Endocrine System: Rare:
Hematologic and Lymphatic System: Infrequent: Rare:
Metabolic and Nutritional Disorders: Infrequent: Rare:
Musculoskeletal System : Infrequent: Rare:
Nervous System: Frequent: Infrequent:Rare:
Respiratory System: Infrequent: Rare:
Special Senses: Frequent: Infrequent:Rare:
Urogenital System: Infrequent: Rare:
6.3. Postmarketing Experience
Blood and Lymphatic:
Gastrointestinal:
Hepatobiliary Tract and Pancreas:
Immunologic:
Lower Respiratory:
Musculoskeletal:
Neurology:
Non-site Specific:
7. DRUG INTERACTIONS
Significant drug interactions with lamotrigine are summarized in Table 13. Additional details of these drug interaction studies are provided in the Clinical Pharmacology subsection [see Clinical Pharmacology (12.3)].
Table 13 Established and Other Potentially Significant Drug Interactions
| Concomitant Drug |
Effect on Concentration of Lamotrigine or Concomitant Drug |
Clinical Comment |
| Estrogen-containing oral contraceptive preparation containing 30 mcg ethinylestradiol and 150 mcg levonorgestrel |
↓ lamotrigine ↓ levonorgestrel |
Decreased lamotrigine levels approximately 50%. Decrease in levonorgestrel component by 19%. |
|
Carbamazepine (CBZ) and CBZ epoxide |
↓ lamotrigine ? CBZ epoxide |
Addition of carbamazepine decreases lamotrigine concentration approximately 40% May increase CBZ epoxide levels |
| Phenobarbital/Primidone |
↓ lamotrigine |
Decreased lamotrigine concentration approximately 40%. |
| Phenytoin (PHT) |
↓ lamotrigine |
Decreased lamotrigine concentration approximately 40%. |
| Rifampin |
↓ lamotrigine |
Decreased lamotrigine AUC approximately 40%. |
| Valproate |
↑ lamotrigine ? valproate |
Increased lamotrigine concentrations slightly more than 2-fold. Decreased valproate concentrations an average of 25% over a 3-week period then stabilized in healthy volunteers; no change in controlled clinical trials in epilepsy patients. |
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Teratogenic Effects:
2
2
2
2
Non-Teratogenic Effects:
Pregnancy Exposure Registry:
http://www.aedpregnancyregistry.org/.
Physicians are also encouraged to register patients in the Lamotrigine Pregnancy Registry; enrollment in this registry must be done prior to any prenatal diagnostic tests and before fetal outcome is known. Physicians can obtain information by calling the Lamotrigine Pregnancy Registry at 1-800-336-2176 (toll-free).
8.2. Labor and Delivery
8.3. Nursing Mothers
8.4. Pediatric Use
8.5. Geriatric Use
8.6. Patients With Hepatic Impairment
Experience in patients with hepatic impairment is limited. Based on a clinical pharmacology study in 24 patients with mild, moderate, and severe liver impairment [see Clinical Pharmacology (12.3)], the following general recommendations can be made. No dosage adjustment is needed in patients with mild liver impairment. Initial, escalation, and maintenance doses should generally be reduced by approximately 25% in patients with moderate and severe liver impairment without ascites and 50% in patients with severe liver impairment with ascites. Escalation and maintenance doses may be adjusted according to clinical response [see Dosage and Administration (2.1)].
8.7. Patients With Renal Impairment
[see Clinical Pharmacology (12.3)]
[see Dosage and Administration (2.1)]
10. OVERDOSAGE
10.1. Human Overdose Experience
Overdoses involving quantities up to 15 g have been reported for lamotrigine tablets, some of which have been fatal. Overdose has resulted in ataxia, nystagmus, increased seizures, decreased level of consciousness, coma, and intraventricular conduction delay.
10.2. Management of Overdose
[see Clinical Pharmacology (12.3)]
11. DESCRIPTION
as9752a
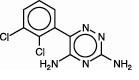
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
The precise mechanism(s) by which lamotrigine exerts its anticonvulsant action are unknown. In animal models designed to detect anticonvulsant activity, lamotrigine was effective in preventing seizure spread in the maximum electroshock (MES) and pentylenetetrazol (scMet) tests, and prevented seizures in the visually and electrically evoked after-discharge (EEAD) tests for antiepileptic activity. Lamotrigine also displayed inhibitory properties in the kindling model in rats both during kindling development and in the fully kindled state. The relevance of these models to human epilepsy, however, is not known.
One proposed mechanism of action of lamotrigine, the relevance of which remains to be established in humans, involves an effect on sodium channels. In vitro pharmacological studies suggest that lamotrigine inhibits voltage-sensitive sodium channels, thereby stabilizing neuronal membranes and consequently modulating presynaptic transmitter release of excitatory amino acids (e.g., glutamate and aspartate).
Although the relevance for human use is unknown, the following data characterize the performance of lamotrigine in receptor binding assays. Lamotrigine had a weak inhibitory effect on the serotonin 5-HT3 receptor (IC50 = 18 μM). It does not exhibit high affinity binding (IC50>100 μM) to the following neurotransmitter receptors: adenosine A1 and A2; adrenergic α1, α2, and β; dopamine D1 and D2; γ-aminobutyric acid (GABA) A and B; histamine H1; kappa opioid; muscarinic acetylcholine; and serotonin 5-HT2. Studies have failed to detect an effect of lamotrigine on dihydropyridine-sensitive calcium channels. It had weak effects at sigma opioid receptors (IC50 = 145 μM). Lamotrigine did not inhibit the uptake of norepinephrine, dopamine, or serotonin, (IC50>200 μM) when tested in rat synaptosomes and/or human platelets in vitro.
Effect of Lamotrigine on N-Methyl d-Aspartate-Receptor Mediated Activity:
Lamotrigine did not inhibit N-methyl d-aspartate (NMDA)-induced depolarizations in rat cortical slices or NMDA-induced cyclic GMP formation in immature rat cerebellum, nor did lamotrigine displace compounds that are either competitive or noncompetitive ligands at this glutamate receptor complex (CNQX, CGS, TCHP). The IC50 for lamotrigine effects on NMDA-induced currents (in the presence of 3 μM of glycine) in cultured hippocampal neurons exceeded 100 μM.
The mechanisms by which lamotrigine exerts its therapeutic action in Bipolar Disorder have not been established.
12.2. Pharmacodynamics
Folate Metabolism:[see Use in Specific Populations (8.1)]
Accumulation in Kidneys:
Melanin Binding:
Cardiovascular:[see Clinical Pharmacology (12.3)]
12.3. Pharmacokinetics
Table 14
Mean a Pharmacokinetic Parameters in Healthy Volunteers and Adult Patients with Epilepsy
| Adult Study Population |
Number of Subjects |
Tmax: Time of Maximum Plasma Concentration (h) |
t½: Elimination Half-life (h) |
Cl/F: Apparent Plasma Clearance (mL/min/kg) |
|
Healthy volunteers taking no other medications:
Single-dose Lamotrigine Tablets Multiple-dose Lamotrigine Tablets |
179 36 |
2.2 (0.25-12.0) 1.7 (0.5-4.0) |
32.8 (14.0-103.0) 25.4 (11.6-61.6) |
0.44 (0.12-1.10) 0.58 (0.24-1.15) |
|
Healthy volunteers taking valproate:
Single-dose Lamotrigine Tablets Multiple-dose Lamotrigine Tablets |
6 18 |
1.8 (1.0-4.0) 1.9 (0.5-3.5) |
48.3 (31.5-88.6) 70.3 (41.9-113.5) |
0.30 (0.14-0.42) 0.18 (0.12-0.33) |
|
Patients with epilepsy taking valproate only:
Single-dose Lamotrigine Tablets |
4 |
4.8 (1.8-8.4) |
58.8 (30.5-88.8) |
0.28 (0.16-0.40) |
|
Patients with epilepsy taking carbamazepine, phenytoin, phenobarbital, or primidoneb plus valproate:
Single-dose Lamotrigine Tablets |
25 |
3.8 (1.0-10.0) |
27.2 (11.2-51.6) |
0.53 (0.27-1.04) |
|
Patients with epilepsy taking carbamazepine, phenytoin, phenobarbital, or primidoneb:
Single-dose Lamotrigine Tablets Multiple-dose Lamotrigine Tablets |
24 17 |
2.3 (0.5-5.0) 2.0 (0.75-5.93) |
14.4 (6.4-30.4) 12.6 (7.5-23.1) |
1.10 (0.51-2.22) 1.21 (0.66-1.82) |
a max
b [see Drug Interactions (7)]
Absorption:
Dose Proportionality:
Distribution:
Protein Binding:
Metabolism:14
Enzyme Induction:
Following multiple administrations (150 mg twice daily) to normal volunteers taking no other medications, lamotrigine induced its own metabolism, resulting in a 25% decrease in t½ and a 37% increase in Cl/F at steady state compared with values obtained in the same volunteers following a single dose. Evidence gathered from other sources suggests that self-induction by lamotrigine may not occur when lamotrigine is given as adjunctive therapy in patients receiving enzyme-inducing drugs such as carbamazepine, phenytoin, phenobarbital, primidone, or drugs such as rifampin that induce lamotrigine glucoronidation [see Drug Interactions (7)].
Elimination: The elimination half-life and apparent clearance of lamotrigine following administration of lamotrigine tablets to adult patients with epilepsy and healthy volunteers is summarized in Table 14. Half-life and apparent oral clearance vary depending on concomitant AEDs.
Drug Interactions: The apparent clearance of lamotrigine is affected by the coadministration of certain medications [see Warnings and Precautions (5.9, 5.13), Drug Interactions (7)].
Table 15 Summary of Drug Interactions With Lamotrigine Tablets
| Drug |
Drug Plasma Concentration With Adjunctive Lamotrigine Tabletsa
|
Lamotrigine Plasma Concentration With Adjunctive Drugsb
|
| Oral contraceptives (e.g., ethinylestradiol /levonorgestrel)c
|
↔d
|
↓ |
| Bupropion |
Not assessed |
↔ |
| Carbamazepine (CBZ) |
↔ |
↓ |
| CBZ epoxidee
|
? |
|
| Felbamate |
Not assessed |
↔ |
| Gabapentin |
Not assessed |
↔ |
| Levetiracetam |
↔ |
↔ |
| Lithium |
↔ |
Not assessed |
| Olanzapine |
↔ |
↔f
|
| Oxcarbazepine |
↔ |
↔ |
| 10-monohydroxy oxcarbazepine metabolite g
|
↔ |
|
| Phenobarbital/primidone |
↔ |
↓ |
| Phenytoin (PHT) |
↔ |
↓ |
| Pregabalin |
↔ |
↔ |
| Rifampin |
Not assessed |
↓ |
| Topiramate |
↔h
|
↔ |
| Valproate |
↓ |
↑ |
| Valproate + PHT and/or CBZ |
Not assessed |
↔ |
| Zonisamide |
Not assessed |
↔ |
a
b
c
d
e
f
g
h
Estrogen-Containing Oral Contraceptives:max
Gradual transient increases in lamotrigine plasma levels (approximate 2-fold increase) occurred during the week of inactive hormone preparation (“pill-free” week) for women not also taking a drug that increased the clearance of lamotrigine (carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin that induce lamotrigine glucorinidation [see Drug Interactions (7)]). The increase in lamotrigine plasma levels will be greater if the dose of lamotrigine tablets is increased in the few days before or during the “pill-free” week. Increases in lamotrigine plasma levels could result in dose-dependent adverse reactions.
In the same study, coadministration of lamotrigine tablets (300 mg/day) in 16 female volunteers did not affect the pharmacokinetics of the ethinylestradiol component of the oral contraceptive preparation. There were mean decreases in the AUC and Cmax of the levonorgestrel component of 19% and 12%, respectively. Measurement of serum progesterone indicated that there was no hormonal evidence of ovulation in any of the 16 volunteers, although measurement of serum FSH, LH, and estradiol indicated that there was some loss of suppression of the hypothalamic-pituitary-ovarian axis.
The effects of doses of lamotrigine tablets other than 300 mg/day have not been systematically evaluated in controlled clinical trials.
The clinical significance of the observed hormonal changes on ovulatory activity is unknown. However, the possibility of decreased contraceptive efficacy in some patients cannot be excluded. Therefore, patients should be instructed to promptly report changes in their menstrual pattern (e.g., break-through bleeding).
Dosage adjustments may be necessary for women receiving estrogen-containing oral contraceptive preparations [see Dosage and Administration (2.1)].
Other Hormonal Contraceptives or Hormone Replacement Therapy: The effect of other hormonal contraceptive preparations or hormone replacement therapy on the pharmacokinetics of lamotrigine has not been systematically evaluated. It has been reported that ethinylestradiol, not progestogens, increased the clearance of lamotrigine up to 2-fold, and the progestin-only pills had no effect on lamotrigine plasma levels. Therefore, adjustments to the dosage of lamotrigine tablets in the presence of progestogens alone will likely not be needed.
Bupropion:
Carbamazepine:[see Adverse Reactions (6.1)]
Felbamate:
Folate Inhibitors:
Gabapentin:
Levetiracetam:
Lithium:
Olanzapine:maxmax
max
Oxcarbazepine:max
max
Phenobarbital, Primidone:
Phenytoin:
Pregabalin:
Rifampin:
Topiramate:
Valproate:
Zonisamide: In a study of 18 patients with epilepsy, coadministration of zonisamide (200 to 400 mg/day) with lamotrigine (150 to 500 mg/day for 35 days) for 35 days had no significant effect on the pharmacokinetics of lamotrigine.
Known Inducers or Inhibitors of Glucuronidation:
Other:.
.
Special Populations: Patients Patients With Renal Impairment: [see Dosage and Administration (2.1)].
Hepatic Disease: [see Dosage and Administration (2.1)]
Age: Pediatric Patients: The pharmacokinetics of lamotrigine following a single 2-mg/kg dose were evaluated in 2 studies of pediatric patients (n = 29 for patients 10 months to 5.9 years of age and n = 26 for patients 5 to 11 years of age). Forty-three patients received concomitant therapy with other AEDs and 12 patients received lamotrigine as monotherapy. Lamotrigine pharmacokinetic parameters for pediatric patients are summarized in Table 16.
[see Dosage and Administration (2.2)].
Table 16
Mean Pharmacokinetic Parameters in Pediatric Patients with Epilepsy
| Pediatric Study Population |
Number of Subjects |
Tmax
(h) |
t½
(h) |
Cl/F (mL/min/kg) |
|
Ages 10 months–5.3 years
Patients taking carbamazepine, phenytoin, phenobarbital, or primidonea Patients taking AEDs with no known effect on the apparent clearance of lamotrigine Patients taking valproate only |
10 7 8 |
3.0 (1.0-5.9) 5.2 (2.9-6.1) 2.9 (1.0-6.0) |
7.7 (5.7-11.4) 19.0 (12.9-27.1) 44.9 (29.5-52.5) |
3.62 (2.44-5.28) 1.2 (0.75-2.42) 0.47 (0.23-0.77) |
|
Ages 5-11 years
Patients taking carbamazepine, phenytoin, phenobarbital, or primidonea Patients taking carbamazepine, phenytoin, phenobarbital, or primidonea plus valproate Patients taking valproate onlyb |
7 8 3 |
1.6 (1.0-3.0) 3.3 (1.0-6.4) 4.5 (3.0-6.0) |
7.0 (3.8-9.8) 19.1 (7.0-31.2) 65.8 (50.7-73.7) |
2.54 (1.35-5.58) 0.89 (0.39-1.93) 0.24 (0.21-0.26) |
|
Ages 13-18 years
Patients taking carbamazepine, phenytoin, phenobarbital, or primidonea Patients taking carbamazepine, phenytoin, phenobarbital, or primidonea plus valproate Patients taking valproate only |
11 8 4 |
c c c |
c c c |
1.3 0.5 0.3 |
a[see Drug Interactions (7)].
bmax
c
Elderly:
Gender:
Race:
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of carcinogenicity was seen in 1 mouse study or 2 rat studies following oral administration of lamotrigine for up to 2 years at maximum tolerated doses (30 mg/kg per day for mice and 10 to 15 mg/kg per day for rats, doses that are equivalent to 90 mg/m2 and 60 to 90 mg/m2, respectively). Steady-state plasma concentrations ranged from 1 to 4 mcg/mL in the mouse study and 1 to 10 mcg/mL in the rat study. Plasma concentrations associated with the recommended human doses of 300 to 500 mg/day are generally in the range of 2 to 5 mcg/mL, but concentrations as high as 19 mcg/mL have been recorded.
Lamotrigine was not mutagenic in the presence or absence of metabolic activation when tested in 2 gene mutation assays (the Ames test and the in vitro mammalian mouse lymphoma assay). In 2 cytogenetic assays (the in vitro human lymphocyte assay and the in vivo rat bone marrow assay), lamotrigine did not increase the incidence of structural or numerical chromosomal abnormalities.
No evidence of impairment of fertility was detected in rats given oral doses of lamotrigine up to 2.4 times the highest usual human maintenance dose of 8.33 mg/kg per day or 0.4 times the human dose on a mg/m2 basis. The effect of lamotrigine on human fertility is unknown.
14. CLINICAL STUDIES
14.1. Epilepsy
Monotherapy with Lamotrigine Tablets in Adults with Partial Seizures Already Receiving Treatment with Carbamazepine, Phenytoin, Phenobarbital, or Primidone as the Single AED: The effectiveness of monotherapy with lamotrigine tablets was established in a multicenter, double-blind clinical trial enrolling 156 adult outpatients with partial seizures. The patients experienced at least 4 simple partial, complex partial, and/or secondarily generalized seizures during each of 2 consecutive 4-week periods while receiving carbamazepine or phenytoin monotherapy during baseline. Lamotrigine tablets (target dose of 500 mg/day) or valproate (1,000 mg/day) was added to either carbamazepine or phenytoin monotherapy over a 4-week period. Patients were then converted to monotherapy with lamotrigine tablets or valproate during the next 4 weeks, then continued on monotherapy for an additional 12-week period.
Study endpoints were completion of all weeks of study treatment or meeting an escape criterion. Criteria for escape relative to baseline were: (1) doubling of average monthly seizure count, (2) doubling of highest consecutive 2-day seizure frequency, (3) emergence of a new seizure type (defined as a seizure that did not occur during the 8-week baseline) that is more severe than seizure types that occur during study treatment or (4) clinically significant prolongation of generalized tonic-clonic (GTC) seizures. The primary efficacy variable was the proportion of patients in each treatment group who met escape criteria.
The percentage of patients who met escape criteria were 42% (32/76) in the lamotrigine tablet group and 69% (55/80) in the valproate group. The difference in the percentage of patients meeting escape criteria was statistically significant (p = 0.0012) in favor of lamotrigine tablet. No differences in efficacy based on age, sex, or race were detected.
Patients in the control group were intentionally treated with a relatively low dose of valproate; as such, the sole objective of this study was to demonstrate the effectiveness and safety of monotherapy with lamotrigine tablets, and cannot be interpreted to imply the superiority of lamotrigine tablet to an adequate dose of valproate.
Adjunctive Therapy with Lamotrigine Tablets in Adults with Partial Seizures: The effectiveness of lamotrigine tablets as adjunctive therapy (added to other AEDs) was established in 3 multicenter, placebo-controlled, double-blind clinical trials in 355 adults with refractory partial seizures. The patients had a history of at least 4 partial seizures per month in spite of receiving one or more AEDs at therapeutic concentrations and, in 2 of the studies, were observed on their established AED regimen during baselines that varied between 8 to 12 weeks. In the third, patients were not observed in a prospective baseline. In patients continuing to have at least 4 seizures per month during the baseline, lamotrigine tablets or placebo was then added to the existing therapy. In all 3 studies, change from baseline in seizure frequency was the primary measure of effectiveness. The results given below are for all partial seizures in the intent-to-treat population (all patients who received at least one dose of treatment) in each study, unless otherwise indicated. The median seizure frequency at baseline was 3 per week while the mean at baseline was 6.6 per week for all patients enrolled in efficacy studies.
One study (n = 216) was a double-blind, placebo-controlled, parallel trial consisting of a 24-week treatment period. Patients could not be on more than 2 other anticonvulsants and valproate was not allowed. Patients were randomized to receive placebo, a target dose of 300 mg/day of lamotrigine tablets, or a target dose of 500 mg/day of lamotrigine tablets. The median reductions in the frequency of all partial seizures relative to baseline were 8% in patients receiving placebo, 20% in patients receiving 300 mg/day of lamotrigine tablets, and 36% in patients receiving 500 mg/day of lamotrigine tablets. The seizure frequency reduction was statistically significant in the 500-mg/day group compared with the placebo group, but not in the 300-mg/day group.
A second study (n = 98) was a double-blind, placebo-controlled, randomized, crossover trial consisting of two 14-week treatment periods (the last 2 weeks of which consisted of dose tapering) separated by a 4-week washout period. Patients could not be on more than 2 other anticonvulsants and valproate was not allowed. The target dose of lamotrigine tablets was 400 mg/day. When the first 12 weeks of the treatment periods were analyzed, the median change in seizure frequency was a 25% reduction on lamotrigine tablets compared to placebo (p<0.001).
The third study (n = 41) was a double-blind, placebo-controlled, crossover trial consisting of two 12-week treatment periods separated by a 4-week washout period. Patients could not be on more than 2 other anticonvulsants. Thirteen patients were on concomitant valproate; these patients received 150 mg/day of lamotrigine tablets. The 28 other patients had a target dose of 300 mg/day of lamotrigine tablets. The median change in seizure frequency was a 26% reduction on lamotrigine tablets compared with placebo (p<0.01).
No differences in efficacy based on age, sex, or race, as measured by change in seizure frequency, were detected.
Adjunctive Therapy with Lamotrigine Tablets in Pediatric Patients with Partial Seizures: The effectiveness of lamotrigine tablets as adjunctive therapy in pediatric patients with partial seizures was established in a multicenter, double-blind, placebo-controlled trial in 199 patients aged 2 to 16 years of age (n = 98 on lamotrigine tablets, n = 101 on placebo). Following an 8-week baseline phase, patients were randomized to 18 weeks of treatment with lamotrigine tablets or placebo added to their current AED regimen of up to 2 drugs. Patients were dosed based on body weight and valproate use. Target doses were designed to approximate 5 mg/kg per day for patients taking valproate (maximum dose: 250 mg/day) and 15 mg/kg per day for the patients not taking valproate (maximum dose: 750 mg per day). The primary efficacy endpoint was percentage change from baseline in all partial seizures. For the intent-to-treat population, the median reduction of all partial seizures was 36% in patients treated with lamotrigine tablets and 7% on placebo, a difference that was statistically significant (p<0.01).
Adjunctive Therapy With Lamotrigine Tablets in Pediatric and Adult Patients With Lennox-Gastaut Syndrome:
Adjunctive Therapy With Lamotrigine Tablets in Pediatric and Adult Patients With Primary Generalized Tonic-Clonic Seizures:
p
14.2. Bipolar Disorder
16. HOW SUPPLIED/STORAGE AND HANDLING
Lamotrigine Tablets, 25 mg
White to off-white, capsule shaped tablets, with break line on one side and debossed with “L121” on other side. They are supplied as follows:
Lamotrigine Tablets, 100 mg
White to off-white, round tablets with break line on one side and debossed with “L122” on other side. They are supplied as follows:
Lamotrigine Tablets, 150 mg
White to off-white, round tablets with break line on one side and debossed with “L123” on other side. They are supplied as follows:
Lamotrigine Tablets, 200 mg
White to off-white, round tablets with break line on one side and debossed with “L124” on other side. They are supplied as follows:
Store at 20º-25°C (68º-77°F); excursion permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
17. PATIENT COUNSELING INFORMATION
17.1. Rash
17.2. Suicidal Thinking and Behavior
17.3. Worsening of Seizures
Patients should be advised to notify their physician if worsening of seizure control occurs.
17.4. CNS Adverse Effects
17.5. Blood Dyscrasias and/or Acute Multiorgan Failure
[see Warnings and Precautions (5.3, 5.4)].
17.6. Pregnancy
Patients should be advised to notify their physicians if they become pregnant or intend to become pregnant during therapy. Patients should be advised to notify their physicians if they intend to breastfeed or are breastfeeding an infant.
[see Use in Specific Populations (8.1)]17.7. Oral Contraceptive Use
[see Warnings and Precautions (5.9), Clinical Pharmacology (12.3)]
17.8. Discontinuing Lamotrigine Tablets
Patients should be advised to notify their physician if they stop taking lamotrigine tablets for any reason and not to resume lamotrigine tablets without consulting their physician.
17.9. Aseptic Meningitis
Patients should be advised that lamotrigine may cause aseptic meningitis. Patients should be advised to notify their physician immediately if they develop signs and symptoms of meningitis such as headache, fever, nausea, vomiting, stiff neck, rash, abnormal sensitivity to light, myalgia, chills, confusion, or drowsiness while taking lamotrigine.
17.10. Potential Medication Errors
To avoid a medication error of using the wrong drug or formulation, patients should be strongly advised to visually inspect their tablets to verify that they are lamotrigine tablets, as well as the correct formulation of lamotrigine tablets, each time they fill their prescription [see Dosage Forms and Strengths (3.1,3.2, 3.3), How Supplied/Storage and Handling (16)]
MEDICATION GUIDE
LAMOTRIGINE TABLETS
Rx only
Read this Medication Guide before you start taking lamotrigine tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment. If you have questions about lamotrigine tablets, ask your healthcare provider or pharmacist.
What is the most important information I should know about lamotrigine tablets?
- Lamotrigine tablets may cause a serious skin rash that may cause you to be hospitalized or to stop lamotrigine tablets; it may rarely cause death.
- take lamotrigine tablets while taking valproate (DEPAKENE (valproic acid) or DEPAKOTE (divalproex sodium)).
- take a higher starting dose of lamotrigine tablets than your healthcare provider prescribed.
- increase your dose of lamotrigine tablets faster than prescribed.
Lamotrigine tablets can also cause other types of allergic reactions or serious problems which may affect organs and other parts of your body like the liver or blood cells. You may or may not have a rash with these types of reactions.
Call your healthcare provider right away if you have any of the following:
- a skin rash
- hives
- fever
- swollen lymph glands
- painful sores in the mouth or around your eyes
- swelling of your lips or tongue
- yellowing of your skin or eyes
- unusual bruising or bleeding
- severe fatigue or weakness
- severe muscle pain
- frequent infections
These symptoms may be the first signs of a serious reaction. A healthcare provider should examine you to decide if you should continue taking lamotrigine tablets.
2. Like other antiepileptic drugs, lamotrigine tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempt to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
Do not stop lamotrigine tablets without first talking to a healthcare provider.
- Stopping lamotrigine tablets suddenly can cause serious problems.
- Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms
3. Lamotrigine tablets may rarely cause aseptic meningitis, a serious inflammation of the protective membrane that covers the brain and spinal cord.
- Headache
- Fever
- Nausea
- Vomiting
- Stiff neck
- Rash
- Unusual sensitivity to light
- Muscle pain
- Chills
- Confusion
- Drowsiness
Lamotrigine tabletscan have other serious side effects.
Taking the wrong medication can cause serious health problems. When your healthcare provider gives you a prescription for lamotrigine tablets:
- Make sure you can read it clearly.
- Talk to your pharmacist to check that you are given the correct medicine.
- Each time you fill your prescription, check the tablets you receive against the pictures of the tablet below.

What is lamotrigine tablet?
- together with other medicines to treat certain types of seizures (partial seizures, primary generalized tonic-clonic seizures, generalized seizures of Lennox-Gastaut syndrome) in people 2 years or older.
- alone when changing from other medicines used to treat partial seizures in people 16 years or older.
- for the long-term treatment of Bipolar I Disorder to lengthen the time between mood episodes in people 18 years or older who have been treated for mood episodes with other medicine.
It is not known if lamotrigine tablet is safe or effective in children or teenagers under the age of 18 with mood disorders such as bipolar disorder or depression.
It is not known if lamotrigine tablet is safe or effective when used alone as the first treatment of seizures in adults.
Who should not take lamotrigine tablet ?
You should not take lamotrigine tablet if you have had an allergic reaction to lamotrigine or to any of the inactive ingredients in lamotrigine tablet. See the end of this leaflet for a complete list of ingredients in lamotrigine tablet.
What should I tell my healthcare provider before taking lamotrigine tablet?
Before taking lamotrigine tablet, tell your healthcare provider about all of your medical conditions, including if you:
- have had a rash or allergic reaction to another antiseizure medicine.
- have or have had depression, mood problems or suicidal thoughts or behavior.
- are taking oral contraceptives (birth control pills) or other female hormonal medicines. Do not start or stop taking birth control pills or other female hormonal medicine until you have talked with your healthcare provider. Tell your healthcare provider if you have any changes in your menstrual pattern such as breakthrough bleeding. Stopping or starting these medicines may cause side effects (such as dizziness, lack of coordination, or double vision) or lessen how well lamotrigine tablet works.
- are pregnant or plan to become pregnant. It is not known if lamotrigine tablet will harm your unborn baby. If you become pregnant while taking lamotrigine tablet, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- are breastfeeding. Lamotrigine tablet can pass into your breast milk. You and your healthcare provider should decide if you should take lamotrigine tablet or breastfeed. Breastfeeding while taking lamotrigine tablet is not recommended.
Tell your healthcare provider about all the medicines you take or if you are planning to take a new medicine, including prescription and non-prescription medicines, vitamins, and herbal supplements. Using lamotrigine tablet with certain other medicines can affect each other, causing side effects.
How should I take lamotrigine tablet?
- Take lamotrigine tablet exactly as prescribed.
- Your healthcare provider may change your dose. Do not change your dose without talking to your healthcare provider.
- Do not stop taking lamotrigine tablet without talking to your healthcare provider. Stopping lamotrigine tablet suddenly may cause serious problems. For example, if you have epilepsy and you stop taking lamotrigine tablet suddenly, you may get seizures that do not stop. Talk with your healthcare provider about how to stop lamotrigine tablet slowly.
- If you miss a dose of lamotrigine tablet, take it as soon as you remember. If it is almost time for your next dose, just skip the missed dose. Take the next dose at your regular time. Do not take two doses at the same time.
- You may not feel the full effect of lamotrigine tablet for several weeks.
- If you have epilepsy, tell your healthcare provider if your seizures get worse or if you have any new types of seizures.
- Swallow lamotrigine tablet whole.
What should I avoid while taking lamotrigine tablet ?
- Do not drive a car or operate complex, hazardous machinery until you know how lamotrigine tablet affects you.
What are possible side effects of lamotrigine tablet?
- See “What is the most important information I should know about lamotrigine tablet?”
Common side effects of lamotrigine tablet include:
- dizziness
- tremor
- headache
- rash
- blurred or double vision
- fever
- lack of coordination
- abdominal pain
- sleepiness
- back pain
- nausea, vomiting
- tiredness
- insomnia
- dry mouth
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of lamotrigine tablet. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store lamotrigine tablet ?
- Store lamotrigine tablet at 20º-25°C (68º-77°F); excursion permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
- Keep lamotrigine tablet and all medicines out of the reach of children.
General information about lamotrigine tablet
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use lamotrigine tablet for a condition for which it was not prescribed. Do not give lamotrigine tablet to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about lamotrigine tablet. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about lamotrigine tablet that is written for healthcare professionals.
What are the ingredients in lamotrigine tablet ?
Lamotrigine tablets
This Medication Guide has been approved by the U.S. Food and Drug Administration.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL




LamotrigineLamotrigine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
LamotrigineLamotrigine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
LamotrigineLamotrigine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
LamotrigineLamotrigine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||