Lamotrigine
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LAMOTRIGINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS AND DRUG METABOLISM
- CLINICAL STUDIES
- INDICATIONS & USAGE
- LAMOTRIGINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LAMOTRIGINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
SERIOUS RASHES REQUIRING HOSPITALIZATION AND DISCONTINUATION OF TREATMENT HAVE BEEN REPORTED IN ASSOCIATION WITH THE USE OF LAMOTRIGINE. THE INCIDENCE OF THESE RASHES, WHICH HAVE INCLUDED STEVENS-JOHNSON SYNDROME, IS APPROXIMATELY 0.8% (8 PER 1,000) IN PEDIATRIC PATIENTS (AGE <16 YEARS) RECEIVING LAMOTRIGINE AS ADJUNCTIVE THERAPY FOR EPILEPSY AND 0.3% (3 PER 1,000) IN ADULTS ON ADJUNCTIVE THERAPY FOR EPILEPSY. IN CLINICAL TRIALS OF BIPOLAR AND OTHER MOOD DISORDERS, THE RATE OF SERIOUS RASH WAS 0.08% (0.8 PER 1,000) IN ADULT PATIENTS RECEIVING LAMOTRIGINE AS INITIAL MONOTHERAPY AND 0.13% (1.3 PER 1,000) IN ADULT PATIENTS RECEIVING LAMOTRIGINE AS ADJUNCTIVE THERAPY. IN A PROSPECTIVELY FOLLOWED COHORT OF 1,983 PEDIATRIC PATIENTS WITH EPILEPSY TAKING ADJUNCTIVE LAMOTRIGINE, THERE WAS 1 RASH-RELATED DEATH. IN WORLDWIDE POSTMARKETING EXPERIENCE, RARE CASES OF TOXIC EPIDERMAL NECROLYSIS AND/OR RASH-RELATED DEATH HAVE BEEN REPORTED IN ADULT AND PEDIATRIC PATIENTS, BUT THEIR NUMBERS ARE TOO FEW TO PERMIT A PRECISE ESTIMATE OF THE RATE.OTHER THAN AGE, THERE ARE AS YET NO FACTORS IDENTIFIED THAT ARE KNOWN TO PREDICT THE RISK OF OCCURRENCE OR THE SEVERITY OF RASH ASSOCIATED WITH LAMOTRIGINE. THERE ARE SUGGESTIONS, YET TO BE PROVEN, THAT THE RISK OF RASH MAY ALSO BE INCREASED BY (1)
COADMINISTRATION OF LAMOTRIGINE WITH VALPROATE (INCLUDES VALPROIC ACID AND DIVALPROEX SODIUM), (2) EXCEEDING THE RECOMMENDED INITIAL DOSE OF LAMOTRIGINE, OR (3) EXCEEDING THE RECOMMENDED DOSE ESCALATION FOR LAMOTRIGINE. HOWEVER, CASES HAVE BEEN REPORTED IN THE ABSENCE OF THESE FACTORS.
NEARLY ALL CASES OF LIFE-THREATENING RASHES ASSOCIATED WITH LAMOTRIGINE HAVE OCCURRED WITHIN 2 TO 8 WEEKS OF TREATMENT INITIATION. HOWEVER, ISOLATED CASES HAVE BEEN REPORTED AFTER PROLONGED TREATMENT (E.G., 6 MONTHS). ACCORDINGLY, DURATION OF THERAPY CANNOT BE RELIED UPON AS A MEANS TO PREDICT THE POTENTIAL RISK HERALDED BY THE FIRST APPEARANCE OF A RASH.
ALTHOUGH BENIGN RASHES ALSO OCCUR WITH LAMOTRIGINE, IT IS NOT POSSIBLE TO PREDICT RELIABLY WHICH RASHES WILL PROVE TO BE SERIOUS OR LIFE THREATENING. ACCORDINGLY, LAMOTRIGINE SHOULD ORDINARILY BE DISCONTINUED AT THE FIRST SIGN OF RASH, UNLESS THE RASH IS CLEARLY NOT DRUG RELATED. DISCONTINUATION OF TREATMENT MAY NOT PREVENT A RASH FROM BECOMING LIFE THREATENING OR PERMANENTLY DISABLING OR DISFIGURING.
SERIOUS RASHES REQUIRING HOSPITALIZATION AND DISCONTINUATION OF TREATMENT HAVE BEEN REPORTED IN ASSOCIATION WITH THE USE OF LAMOTRIGINE. THE INCIDENCE OF THESE RASHES, WHICH HAVE INCLUDED STEVENS-JOHNSON SYNDROME, IS APPROXIMATELY 0.8% (8 PER 1,000) IN PEDIATRIC PATIENTS (AGE <16 YEARS) RECEIVING LAMOTRIGINE AS ADJUNCTIVE THERAPY FOR EPILEPSY AND 0.3% (3 PER 1,000) IN ADULTS ON ADJUNCTIVE THERAPY FOR EPILEPSY. IN CLINICAL TRIALS OF BIPOLAR AND OTHER MOOD DISORDERS, THE RATE OF SERIOUS RASH WAS 0.08% (0.8 PER 1,000) IN ADULT PATIENTS RECEIVING LAMOTRIGINE AS INITIAL MONOTHERAPY AND 0.13% (1.3 PER 1,000) IN ADULT PATIENTS RECEIVING LAMOTRIGINE AS ADJUNCTIVE THERAPY. IN A PROSPECTIVELY FOLLOWED COHORT OF 1,983 PEDIATRIC PATIENTS WITH EPILEPSY TAKING ADJUNCTIVE LAMOTRIGINE, THERE WAS 1 RASH-RELATED DEATH. IN WORLDWIDE POSTMARKETING EXPERIENCE, RARE CASES OF TOXIC EPIDERMAL NECROLYSIS AND/OR RASH-RELATED DEATH HAVE BEEN REPORTED IN ADULT AND PEDIATRIC PATIENTS, BUT THEIR NUMBERS ARE TOO FEW TO PERMIT A PRECISE ESTIMATE OF THE RATE.
OTHER THAN AGE, THERE ARE AS YET NO FACTORS IDENTIFIED THAT ARE KNOWN TO PREDICT THE RISK OF OCCURRENCE OR THE SEVERITY OF RASH ASSOCIATED WITH LAMOTRIGINE. THERE ARE SUGGESTIONS, YET TO BE PROVEN, THAT THE RISK OF RASH MAY ALSO BE INCREASED BY (1)
COADMINISTRATION OF LAMOTRIGINE WITH VALPROATE (INCLUDES VALPROIC ACID AND DIVALPROEX SODIUM), (2) EXCEEDING THE RECOMMENDED INITIAL DOSE OF LAMOTRIGINE, OR (3) EXCEEDING THE RECOMMENDED DOSE ESCALATION FOR LAMOTRIGINE. HOWEVER, CASES HAVE BEEN REPORTED IN THE ABSENCE OF THESE FACTORS.
NEARLY ALL CASES OF LIFE-THREATENING RASHES ASSOCIATED WITH LAMOTRIGINE HAVE OCCURRED WITHIN 2 TO 8 WEEKS OF TREATMENT INITIATION. HOWEVER, ISOLATED CASES HAVE BEEN REPORTED AFTER PROLONGED TREATMENT (E.G., 6 MONTHS). ACCORDINGLY, DURATION OF THERAPY CANNOT BE RELIED UPON AS A MEANS TO PREDICT THE POTENTIAL RISK HERALDED BY THE FIRST APPEARANCE OF A RASH.
ALTHOUGH BENIGN RASHES ALSO OCCUR WITH LAMOTRIGINE, IT IS NOT POSSIBLE TO PREDICT RELIABLY WHICH RASHES WILL PROVE TO BE SERIOUS OR LIFE THREATENING. ACCORDINGLY, LAMOTRIGINE SHOULD ORDINARILY BE DISCONTINUED AT THE FIRST SIGN OF RASH, UNLESS THE RASH IS CLEARLY NOT DRUG RELATED. DISCONTINUATION OF TREATMENT MAY NOT PREVENT A RASH FROM BECOMING LIFE THREATENING OR PERMANENTLY DISABLING OR DISFIGURING.
LAMOTRIGINE DESCRIPTION
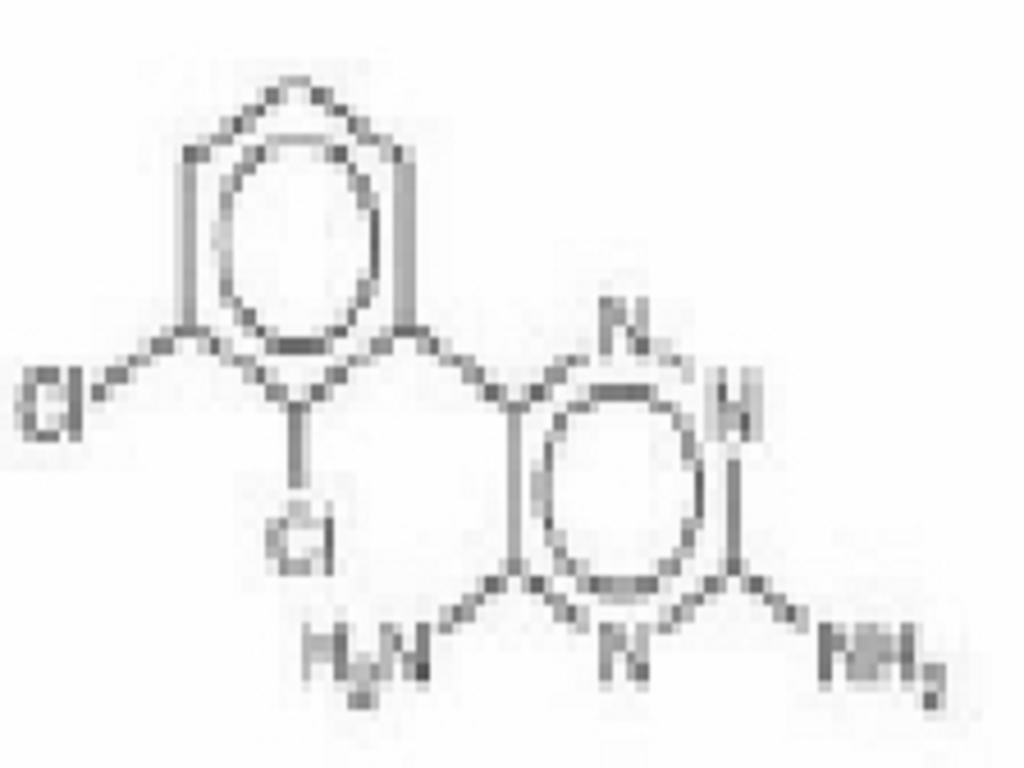
CLINICAL PHARMACOLOGY
Mechanism of ActionPharmacological Properties
Folate Metabolism
PRECAUTIONS: Pregnancy
Accumulation in Kidneys
Melanin Binding
Cardiovascular
Drug Disposition
PHARMACOKINETICS AND DRUG METABOLISM
*
*CLINICAL PHARMACOLOGY: Drug Interactions:PRECAUTIONS: Drug Interactions
Absorption:
Distribution:
Protein Binding:
Drug Disposition:
Drug Interactions:
DOSAGE AND ADMINISTRATIONPRECAUTIONS: Drug Interactions
PRECAUTIONS: Drug Interactions
DOSAGE AND ADMINISTRATIONPRECAUTIONS: Drug Interactions
PRECAUTIONS: Drug Interactions
PRECAUTIONS: Drug Interactions
PRECAUTIONS: Drug Interactions
PRECAUTIONS: Drug Interactions
Table 1
DOSAGE AND ADMINISTRATION: Patient With Hepatic Impairment
Table 2
DOSAGE AND ADMINISTRATION
*CLINICAL PHARMACOLOGY: Drug Interactions:PRECAUTIONS: Drug Interactions*****
CLINICAL STUDIES
INDICATIONS & USAGE
Epilepsy:Adjunctive Use:
Monotherapy Use:
DOSAGE AND ADMINISTRATION
Bipolar Disorder
CLINICAL STUDIES, Bipolar Disorder
LAMOTRIGINE CONTRAINDICATIONS
WARNINGS
SEE BOX WARNING REGARDING THE RISK OF SERIOUS RASHES REQUIRING HOSPITALIZATION AND DISCONTINUATION OF LAMOTRIGINE.ALTHOUGH BENIGN RASHES ALSO OCCUR WITH LAMOTRIGINE, IT IS NOT POSSIBLE TO PREDICT RELIABLY WHICH RASHES WILL PROVE TO BE SERIOUS OR LIFE THREATENING. ACCORDINGLY, LAMOTRIGINE SHOULD ORDINARILY BE DISCONTINUED AT THE FIRST SIGN OF RASH, UNLESS THE RASH IS CLEARLY NOT DRUG RELATED. DISCONTINUATION OF TREATMENT MAY NOT PREVENT A RASH FROM BECOMING LIFE THREATENING OR PERMANENTLY DISABLING OR DISFIGURING.
Serious Rash:
Pediatric Population:
Adult Population:
Hypersensitivity Reactions:
Prior to initiation of treatment with Lamotrigine, the patient should be instructed that a rash or other signs or symptoms of hypersensitivity (e.g., fever, lymphadenopathy) may herald a serious medical event and that the patient should report any such occurrence to a physician immediately.
Acute Multiorgan Failure:
Blood Dyscrasias
Withdrawal Seizures:
DOSAGE AND ADMINISTRATION
PRECAUTIONS
Concomitant Use With Oral Contraceptives:PRECAUTIONS: Drug InteractionsDosage adjustments will be necessary in most patients who start or stop estrogen- containing oral contraceptives while taking LamotrigineDOSAGE AND ADMINISTRATION: Special Populations: Women and Oral Contraceptives: Adjustments to the Maintenance Dose of Lamotrigine
Dermatological Events
BOX WARNINGWARNINGS
ACCORDINGLY, LAMOTRIGINE SHOULD ORDINARILY BE DISCONTINUED AT THE FIRST SIGN OF RASH, UNLESS THE RASH IS CLEARLY NOT DRUG RELATED. DISCONTINUATION OF TREATMENT MAY NOT PREVENT A RASH FROM BECOMING LIFE THREATENING OR PERMANENTLY DISABLING OR DISFIGURING.
CLINICAL PHARMACOLOGY: Pharmacokinetics and Drug MetabolismDOSAGE AND ADMINISTRATION
Use in Patients With Epilepsy:
Sudden Unexplained Death in Epilepsy (SUDEP)
Status Epilepticus:
Use in Patients With Bipolar Disorder:
Acute Treatment of Mood Episodes:
Children and Adolescents (less than 18 years of age):
PRECAUTIONS: Clinical Worsening and Suicide Risk Associated with Bipolar Disorder:
Clinical Worsening and Suicide Risk Associated with Bipolar Disorder:
OVERDOSAGE
Addition of Lamotrigine to a Multidrug Regimen That Includes Valproate (Dosage Reduction)
DOSAGE AND ADMINISTRATION
Use in Patients With Concomitant Illness:
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
Binding in the Eye and Other Melanin-Containing Tissues:
INFORMATION FOR PATIENTS
PRECAUTIONS: Drug Interactions
PATIENT INFORMATION
Phenylketonurics:
LABORATORY TESTS
DRUG INTERACTIONS
DOSAGE AND ADMINISTRATIONOral Contraceptives:
PRECAUTIONS: Concomitant Use With Oral Contraceptives
DOSAGE AND ADMINISTRATION: Special Populations: Women and Oral Contraceptives
ADVERSE REACTIONS
CLINICAL PHARMACOLOGY: Pharmacokinetics and Drug MetabolismCLINICAL PHARMACOLOGY
*PRECAUTIONS: Drug Interactions: Oral Contraceptives#*#
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic effects: Pregnancy Category CNon-Teratogenic Effects:
Pregnancy Exposure Registry:
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
LAMOTRIGINE ADVERSE REACTIONS
SERIOUS RASH REQUIRING HOSPITALIZATION AND DISCONTINUATION OF LAMOTRIGINE, INCLUDING STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS, HAVE OCCURRED IN ASSOCIATION WITH THERAPY WITH LAMOTRIGINE. RARE DEATHS HAVE BEEN REPORTED, BUT THEIR NUMBERS ARE TOO FEW TO PERMIT A PRECISE ESTIMATE OF THE RATE (seeBOX WARNING).Epilepsy:
Most Common Adverse Events in All Clinical Studies:
Adjunctive Therapy in Adults With Epilepsy:
WARNINGS
Monotherapy in Adults With Epilepsy:
Adjunctive Therapy in Pediatric Patients With Epilepsy:
Incidence in Controlled Clinical Studies of Epilepsy:
Incidence in Controlled Adjunctive Clinical Studies in Adults With Epilepsy:
Table 4. Treatment-Emergent Adverse Event Incidence in Placebo-Controlled Adjunctive Trials in Adult Patients With Epilepsy*(Events in at least 2% of patients treated with lamotrigine and numerically more frequent than in the placebo group.)
*Adverse experiences reported by at least 2% of patients treated with Lamotrigine are included.Body System/Adverse ExperiencePercent of Patients Receiving Adjunctive Lamotrigine (n = 711)Percent of Patients Receiving Adjunctive Placebo (n = 419)Body as a wholeHeadache2919Flu syndrome76Fever64Abdominal pain54Neck pain21Reaction aggravated (seizure exacerbation)21DigestiveNausea1910Vomiting94Diarrhea64Dyspepsia52Constipation43Tooth disorder32Anorexia21MusculoskeletalArthralgia20Nervous Dizziness3813Ataxia226Somnolence147Incoordination62Insomnia62Tremor41Depression43Anxiety43Convulsion31Irritability32Speech disorder30Concentration disturbance21RespiratoryRhinitis149Pharyngitis109Cough increased86Skin and appendagesRash105Pruritus32Special sensesDiplopia287Blurred vision165Vision abnormality31UrogenitalFemale patients only(n = 365)(n = 207)Dysmenorrhea76Vaginitis41Amenorrhea21In a randomized, parallel study comparing placebo and 300 and 500 mg/day of Lamotrigine, some of the more common drug-related adverse events were dose related (see Table 5).
Table 5. Dose-Related Adverse Events From a Randomized, Placebo-Controlled Trial in Adults With Epilepsy
*Significantly greater than placebo group (p<0.05).Significantly greater than group receiving Lamotrigine 300 mg (p<0.05).Percent of Patients Experiencing Adverse ExperiencesAdverse ExperiencePlacebo (n = 73)Lamotrigine 300 mg (n = 71)Lamotrigine 500 mg (n = 72)Ataxia Blurred vision Diplopia Dizziness Nausea Vomiting10 10 8 27 11 410 11 24*31 18 1128*25*49*54*25*18*Other events that occurred in more than 1% of patients but equally or more frequently in the placebo group included: asthenia, back pain, chest pain, flatulence, menstrual disorder, myalgia, paresthesia, respiratory disorder, and urinary tract infection.
The overall adverse experience profile for lamotrigine was similar between females and males, and was independent of age. Because the largest non-Caucasian racial subgroup was only 6% of patients exposed to Lamotrigine in placebo-controlled trials, there are insufficient data to support a statement regarding the distribution of adverse experience reports by race. Generally, females receiving either adjunctive lamotrigine or placebo were more likely to report adverse experiences than males. The only adverse experience for which the reports on lamotrigine were greater than 10% more frequent in females than males (without a corresponding difference by gender on placebo) was dizziness (difference = 16.5%). There was little difference between females and males in the rates of discontinuation of lamotrigine for individual adverse experiences.
Incidence in a Controlled Monotherapy Trial in Adults With Partial Seizures:
Table 6 lists treatment-emergent signs and symptoms that occurred in at least 5% of patients with epilepsy treated with monotherapy with Lamotrigine in a double-blind trial following discontinuation of either concomitant carbamazepine or phenytoin not seen at an equivalent frequency in the control group.
Table 6. Treatment-Emergent Adverse Event Incidence in Adults With Partial Seizures in a Controlled Monotherapy Trial*(Events in at least 5% of patients treated with lamotrigine and numerically more frequent than in the valproate group.)
*Adverse experiences reported by at least 5% of patients are included.Up to 500 mg/day.1,000 mg/day.Body System/Adverse ExperiencePercent of Patients Receiving Lamotrigine Monotherapy(n = 43)Percent of Patients Receiving Low- Dose Valproate(n = 44)Body as a wholePain50Infection52Chest pain52DigestiveVomiting90Dyspepsia72Nausea72Metabolic and nutritionalWeight decrease52Nervous Coordination abnormality70Dizziness70Anxiety50Insomnia52RespiratoryRhinitis72Urogenital (female patients only)(n = 21)(n = 28)Dysmenorrhea50Adverse events that occurred with a frequency of less than 5% and greater than 2% of patients receiving Lamotrigine and numerically more frequent than placebo were:
Body as a Whole:Asthenia, fever.
Digestive: Anorexia, dry mouth, rectal hemorrhage, peptic ulcer.
Metabolic and Nutritional:Peripheral edema.
Nervous System:Amnesia, ataxia, depression, hypesthesia, libido increase, decreased reflexes, increased reflexes, nystagmus, irritability, suicidal ideation.
Respiratory:Epistaxis, bronchitis, dyspnea.
Skin and Appendages:Contact dermatitis, dry skin, sweating.
Special Senses:Vision abnormality.
Incidence in Controlled Adjunctive Trials in Pediatric Patients With Epilepsy:
Table 7 lists adverse events that occurred in at least 2% of 339 pediatric patients with partial seizures or generalized seizures of Lennox-Gastaut syndrome, who received Lamotrigine up to 15 mg/kg per day or a maximum of 750 mg per day. Reported adverse events were classified using COSTART terminology.
Table 7. Treatment-Emergent Adverse Event Incidence in Placebo-Controlled Adjunctive Trials in Pediatric Patients With Epilepsy (Events in at least 2% of patients treated with lamotrigine and numerically more frequent than in the placebo group.)
Body System/ Adverse ExperiencePercent of Patients Receiving Lamotrigine (n = 168)Percent of Patients Receiving Placebo (n = 171)Body as a whole Infection2017Fever1514Accidental injury1412Abdominal pain105Asthenia84Flu syndrome76Pain54Facial edema21Photosensitivity20CardiovascularHemorrhage21DigestiveVomiting2016Diarrhea119Nausea102Constipation42Dyspepsia21Tooth disorder21Hemic and lymphaticLymphadenopathy21Metabolic and nutritionalEdema20Nervous system Somnolence1715Dizziness144Ataxia113Tremor101Emotional lability42Gait abnormality42Thinking abnormality32Convulsions21Nervousness21Vertigo21RespiratoryPharyngitis1411Bronchitis75Increased cough76Sinusitis21Bronchospasm21SkinRash1412Eczema21Pruritus21Special sensesDiplopia51Blurred vision41Ear disorder21Visual abnormality20UrogenitalMale and female patients Urinary tract infection0Male patients onlyn=92 0 -Bipolar Disorder
The most commonly observed (adverse experiences seen in association with the use of lamotrigine as monotherapy (100 to 400 mg/day) in Bipolar Disorder in the 2 double-blind, placebo-controlled trials of 18 months' duration, and numerically more frequent than in placebo-treated patients are included in Table 8. Adverse events that occurred in at least 5% of patients and were numerically more common during the dose escalation phase of Lamotrigine in these trials (when patients may have been receiving concomitant medications) compared to the monotherapy phase were: headache (25%), rash (11%), dizziness (10%), diarrhea (8%), dream abnormality (6%), and pruritus (6%).
During the monotherapy phase of the double-blind, placebo-controlled trials of 18 months' duration, 13% of 227 patients who received Lamotrigine (100 to 400 mg/day), 16% of 190 patients who received placebo, and 23% of 166 patients who received lithium discontinued therapy because of an adverse experience. The adverse events which most commonly led to discontinuation of Lamotrigine were rash (3%) and mania/hypomania/mixed mood adverse events (2%). Approximately 16% of 2,401 patients who received Lamotrigine (50 to 500 mg/day) for Bipolar Disorder in premarketing trials discontinued therapy because of an adverse experience; most commonly due to rash (5%) and mania/hypomania/mixed mood adverse events (2%).
Incidence in Controlled Clinical Studies of Lamotrigine for the Maintenance Treatment of Bipolar I Disorder
Table 8 lists treatment-emergent signs and symptoms that occurred in at least 5% of patients with Bipolar Disorder treated with Lamotrigine monotherapy (100 to 400 mg/day), following the discontinuation of other psychotropic drugs, in 2 double-blind, placebo- controlled trials of 18 months' duration and were numerically more frequent than in the placebo group.
Table 8. Treatment-Emergent Adverse Event Incidence in 2 Placebo-Controlled Trials in Adults With Bipolar I Disorder*(Events in at least 5% of patients treated with lamotrigine monotherapy and numerically more frequent than in the placebo group.)
*Adverse experiences reported by at least 5% of patients are included.In the overall bipolar and other mood disorders clinical trials, the rate of serious rash was 0.08% (1 of 1,233) of adultpatients who received Lamotrigine as initial monotherapy and 0.13% (2 of 1,538) of adult patients who received lamotrigine as adjunctive therapy (seeWARNINGS).Body System/ Adverse ExperiencePercent of Patients Receiving Lamotrigine n = 227Percent of Patients Receiving Placebo n = 190GeneralBack pain86Fatigue85Abdominal pain63DigestiveNausea1411Constipation52Vomiting52Nervous System Insomnia106Somnolence97Xerostomia (dry mouth)64RespiratoryRhinitis74Exacerbation of cough53Pharyngitis54SkinRash (nonserious)75These adverse events were usually mild to moderate in intensity.
Other events that occurred in 5% or more patients but equally or more frequently in the placebo group included: dizziness, mania, headache, infection, influenza, pain, accidental injury, diarrhea, and dyspepsia.
Adverse events that occurred with a frequency of less than 5% and greater than 1% of patients receiving Lamotrigine and numerically more frequent than placebo were:
General:Fever,neck pain.Cardiovascular:Migraine.Digestive:Flatulence.
Metabolic and Nutritional:Weight gain, edema.
Musculoskeletal:Arthralgia, myalgia.
Nervous System:Amnesia, depression, agitation, emotional lability, dyspraxia, abnormal thoughts, dream abnormality, hypoesthesia.
Respiratory:Sinusitis.
Urogenital:Urinary frequency.
Adverse Events Following Abrupt Discontinuation:
In the 2 maintenance trials, there was no increase in the incidence, severity or type of adverse events in Bipolar Disorder patients after abruptly terminating Lamotrigine therapy. In clinical trials in patients with Bipolar Disorder, 2 patients experienced seizures shortly after abrupt withdrawal of Lamotrigine. However, there were confounding factors that may have contributed to the occurrence of seizures in these bipolar patients (seeDOSAGE AND ADMINISTRATION).
Mania/Hypomania/Mixed Episodes:
During the double-blind, placebo-controlled clinical trials in Bipolar I Disorder in which patients were converted to Lamotrigine monotherapy (100 to 400 mg/day) from other psychotropic medications and followed for durations up to 18 months, the rate of manic or hypomanic or mixed mood episodes reported as adverse experiences was 5% for patients treated with Lamotrigine (n = 227), 4% for patients treated with lithium (n = 166), and 7% for patients treated with placebo (n = 190). In all bipolar controlled trials combined, adverse events of mania (including hypomania and mixed mood episodes) were reported in 5% of patients treated with lamotrigine (n = 956), 3% of patients treated with lithium (n = 280), and 4% of patients treated with placebo (n = 803). The overall adverse event profile for lamotrigine was similar between females and males, between elderly and nonelderly patients, and among racial groups.
Other Adverse Events Observed During All Clinical Trials For Pediatric and Adult Patients With Epilepsy or Bipolar Disorder and Other Mood Disorders
Lamotrigine has been administered to 6,694 individuals for whom complete adverse event data was captured during all clinical trials, only some of which were placebo controlled. During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using modified COSTART dictionary terminology. The frequencies presented represent the proportion of the 6,694 individuals exposed to Lamotrigine who experienced an event of the type cited on at least one occasion while receiving Lamotrigine. All reported events are included except those already listed in the previous tables or elsewhere in the labeling, those too general to be informative, and those not reasonably associated with the use of the drug. Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1,000 patients; rare adverse events are those occurring in fewer than 1/1,000 patients.
Body as a Whole:
Infrequent:Allergic reaction, chills, halitosis, and malaise.
Rare:Abdomen enlarged, abscess, and suicide/suicide attempt.
Cardiovascular System:
Infrequent:Flushing, hot flashes, hypertension, palpitations, postural hypotension, syncope, tachycardia, and vasodilation.
Rare:Angina pectoris, atrial fibrillation, deep thrombophlebitis, ECG abnormality, and myocardial infarction. Dermatological:
Infrequent:Acne, alopecia, hirsutism, maculopapular rash, skin discoloration, and urticaria.
Rare:Angioedema, erythema, exfoliative dermatitis, fungal dermatitis, herpes zoster, leukoderma, multiforme erythema, petechial rash, pustular rash, seborrhea, Stevens-Johnson syndrome, and vesiculobullous rash.
Digestive System:
Infrequent:Dysphagia, eructation, gastritis, gingivitis, increased appetite, increased salivation, liver function tests abnormal, and mouth ulceration.
Rare:Gastrointestinal hemorrhage, glossitis, gum hemorrhage, gum hyperplasia, hematemesis, hemorrhagic colitis, hepatitis, melena, stomach ulcer, stomatitis, thirst, and tongue edema.
Endocrine System:
Rare:Goiter and hypothyroidism.
Hematologic and Lymphatic System:
Infrequent:Ecchymosis and leukopenia.
Rare:Anemia, eosinophilia, fibrin decrease, fibrinogen decrease, iron deficiency anemia, leukocytosis, lymphocytosis, macrocytic anemia, petechia, and thrombocytopenia.
Metabolic and Nutritional Disorders:
Infrequent: Aspartate transaminase increased.
Rare:Alcohol intolerance, alkaline phosphatase increase, alanine transaminase increase, bilirubinemia, general edema, gamma glutamyl transpeptidase increase, and hyperglycemia.
Musculoskeletal System:
Infrequent:Arthritis, leg cramps, myasthenia, and twitching.
Rare:Bursitis, joint disorder, muscle atrophy, pathological fracture, and tendinous contracture.
Nervous System:
Frequent:Confusion and paresthesia.
Infrequent:Akathisia, apathy, aphasia, CNS depression, depersonalization, dysarthria, dyskinesia, euphoria, hallucinations, hostility, hyperkinesia, hypertonia, libido decreased, memory decrease, mind racing, movement disorder, myoclonus, panic attack, paranoid reaction, personality disorder, psychosis, sleep disorder, stupor, and suicidal ideation.
Rare:Cerebellar syndrome, cerebrovascular accident, cerebral sinus thrombosis, choreoathetosis, CNS stimulation, delirium, delusions, dysphoria, dystonia, extrapyramidal syndrome, faintness, grand mal convulsions, hemiplegia, hyperalgesia, hyperesthesia, hypokinesia, hypotonia, manic depression reaction, muscle spasm, neuralgia, neurosis, paralysis, and peripheral neuritis.
Respiratory System:
Infrequent:Yawn.
Rare:Hiccup and hyperventilation.
Special Senses:
Frequent:Amblyopia.
Infrequent:Abnormality of accommodation, conjunctivitis, dry eyes, ear pain, photophobia, taste perversion, and tinnitus.
Rare:Deafness, lacrimation disorder, oscillopsia, parosmia, ptosis, strabismus, taste loss, uveitis, and visual field defect.
Urogenital System:
Infrequent:Abnormal ejaculation, breast pain, hematuria, impotence, menorrhagia, polyuria, urinary incontinence, and urine abnormality.
Rare:Acute kidney failure, anorgasmia, breast abscess, breast neoplasm, creatinine increase, cystitis, dysuria, epididymitis, female lactation, kidney failure, kidney pain, nocturia, urinary retention, urinary urgency, and vaginal moniliasis.
Postmarketing and Other Experience:
In addition to the adverse experiences reported during clinical testing of Lamotrigine, the following adverse experiences have been reported in patients receiving marketed Lamotrigine and from worldwide noncontrolled investigational use. These adverse experiences have not been listed above, and data are insufficient to support an estimate of their incidence or to establish causation.
Blood and Lymphatic:Agranulocytosis, aplastic anemia, disseminated intravascular coagulation, hemolytic anemia, neutropenia, pancytopenia, red cell aplasia.
Gastrointestinal:Esophagitis.
Hepatobiiary Tract and Pancreas:Pancreatitis.
Immunologic:Lupus-like reaction, vasculitis.
Lower Respiratory:Apnea.
Musculoskeletal:Rhabdomyolysis has been observed in patients experiencing hypersensitivity reactions.
Neurology:Exacerbation of parkinsonian symptoms in patients with pre-existing Parkinson's disease, tics.
Non-site Specific:Hypersensitivity reaction, multiorgan failure, progressive immunosuppression.
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
Human Overdose Experience:Management of Overdose:
CLINICAL PHARMACOLOGY
DOSAGE & ADMINISTRATION
EpilepsyAdjunctive Use:
Monotherapy Use:
Safety and effectiveness of lamotrigine tablets and lamotrigine tablets (chewable, dispersible)have not been established. (1) as initial monotherapy, (2) for conversion to monotherapy from AEDs other than carbamazepine, phenytoin, phenobarbital, primidone, or valproate, or (3) for simultaneous conversion to monotherapy from 2 or more concomitant AEDs.
Bipolar Disorder
General Dosing Considerations for Epilepsy and Bipolar Disorder Patients:
BOX WARNING
Lamotrigine Tablets and Lamotrigine Tablets (Chewable, Dispersible)Added to Drugs Known to Induce or Inhibit Glucuronidation:
PRECAUTIONS: Drug Interactions
Target Plasma Levels for Patients With Epilepsy or Bipolar Disorder:
CLINICAL PHARMACOLOGY: Pharmacokinetics and Drug Metabolism
DOSAGE AND ADMINISTRATION: Special Populations
Special Populations: Women and Oral Contraceptives: Starting Lamotrigine Tablets and LamotrigineTablets (Chewable, Dispersible)in Women Taking Oral Contraceptives
PRECAUTIONS: Drug InteractionsTable 11
Adjustments to the Maintenance Dose of Lamotrigine Tablets and Lamotrigine Tablets (Chewable, Dispersible)
1. Taking Estrogen-Containing Oral Contraceptives:
PRECAUTIONS: Drug InteractionsTable 11, column 2PRECAUTIONS: Drug InteractionsPRECAUTIONS: Drug Interactions
Women and Other Hormonal Contraceptive Preparations or Hormone Replacement Therapy:
Patients With Hepatic Impairment:
CLINICAL PHARMACOLOGY
Patients With Renal Functional Impairment:
CLINICAL PHARMACOLOGY
Epilepsy:
Patients 2 to 12 Years of Age:
Table 9. Escalation Regimen for Lamotrigine in Patients 2 to 12 Years of Age With Epilepsy
*PRECAUTIONS: Drug Interactions**
Table 10. The Initial Weight-Based Dosing Guide for Patients 2 to 12 Years Taking Valproate (Weeks 1 to 4) With Epilepsy
Table 11. Escalation Regimen for Lamotrigine in Patients Over 12 Years of Age With Epilepsy
*PRECAUTIONS: Drug Interactions**Conversion from Adjunctive Therapy With Carbamazepine, Phenytoin, Phenobarbital, Primidone, or Valproate as the Single AED to Monotherapy With Lamotrigine in PatientsYears of Age With Epilepsy:
BOX WARNING
Conversion From Adjunctive Therapy With Carbamazepine, Phenytoin, Phenobarbital, or Primidone to Monotherapy With Lamotrigine:
Conversion from Adjunctive Therapy With Valproate to Monotherapy With LamotrigineTable 12
Table 12. Conversion From Adjunctive Therapy With Valproate to Monotherapy With Lamotrigine in PatientsYears of Age With Epilepsy
Conversion from Adjunctive Therapy With Antiepileptic Drugs Other Than Carbamazepine, Phenytoin, Phenobarbital, Primidone, or Valproate to Monotherapy With Lamotrigine:
Usual Maintenance Dose for Epilepsy
Discontinuation Strategy for Patients With Epilepsy:
PRECAUTIONS
Bipolar Disorder
CLINICAL STUDIES: Bipolar DisorderTable 14
DOSAGE AND ADMINISTRATION: Special Populations: Women and Oral Contraceptives: Adjustments to the Maintenace Dose of Lamotrigine
CLINICAL PHARMACOLOGY: Drug InteractionsBOX WARNING
Table 13. Escalation Regimen for Lamotrigine for Patients With Bipolar Disorder*
*CLINICAL PHARMACOLOGY: Drug Interactions:PRECAUTIONS: Drug Interactions
*
*CLINICAL PHARMACOLOGY: Drug Interactions:PRECAUTIONS: Drug InteractionsCLINICAL STUDIES: Bipolar Disorder
Discontinuation Strategy in Bipolar Disorder:
Administration of Lamotrigine Tablets (Chewable, Dispersible)
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
-
● Most people who take lamotrigine tolerate it well. Common side effects with lamotrigine include dizziness, headache, blurred or doubled vision, lack of coordination, sleepiness, nausea, vomiting, insomnia, and rash. Lamotrigine may cause other side effects not listed in this leaflet. If you develop any side effects or symptoms you are concerned about or need more information, call your doctor.
-
● Although most patients who develop rash while receiving lamotrigine have mild to moderate symptoms, some individuals may develop a seriousskin reaction that requires hospitalization. Rarely, deaths have been reported. These serious skin reactions are most likely to happen within the first 8 weeks of treatment with lamotrigine. Serious skin reactions occur more often in children than in adults.
-
● Rashes may be more likely to occur if you: (1) take lamotrigine in combination with valproate [DEPAKNE (valproic acid) or DEPAKOTE (divalproex sodium)], (2) take a higher starting dose of lamotrigine than your doctor prescribed, or (3) increase your dose of lamotrigine faster than prescribed.
-
● It is not possible to predict whether a mild rash will develop into a more serious reaction.
-
● Do not start or stop using birth control pills or other female hormonal products until you have consulted your doctor. Stopping or starting these products may cause side effects (such as dizziness, lack of coordination, or double vision) or decrease the effectiveness of lamotrigine.
-
● Tell your doctor as soon as possible if you experience side effects or changes in your menstrual pattern (e.g., Break-through bleeding) while taking lamotrigine and birth control pills or other female hormonal products.
-
● It is important to take lamotrigine exactly as instructed by your doctor. The dose of lamotrigine must be increased slowly. It may take several weeks or months before your final dosage can be determined by your doctor, based on your response.
-
● Do not increase your dose of lamotrigine or take more frequent doses than those indicated by your doctor. Contact your doctor, if you stop taking lamotrigine for any reason. Do not restart without consulting your doctor.
-
● If you miss a dose of lamotrigine, do not double your next dose.
-
● Always tell your doctor and pharmacist if you are taking any other prescription or over-the-counter medicines. Tell your doctor before you start any other medicines.
-
● Do NOT stop taking lamotrigine or any of your other medicines unless instructed by your doctor.
-
● Use caution before driving a car or operating complex, hazardous machinery until you know if lamotrigine affects your ability to perform these tasks.
-
● If you have epilepsy, tell your doctor if your seizures get worse or if you have any new types of seizures.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

LamotrigineLamotrigine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!