Labetalol Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- LABETALOL HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- INDICATIONS & USAGE
- LABETALOL HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LABETALOL HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
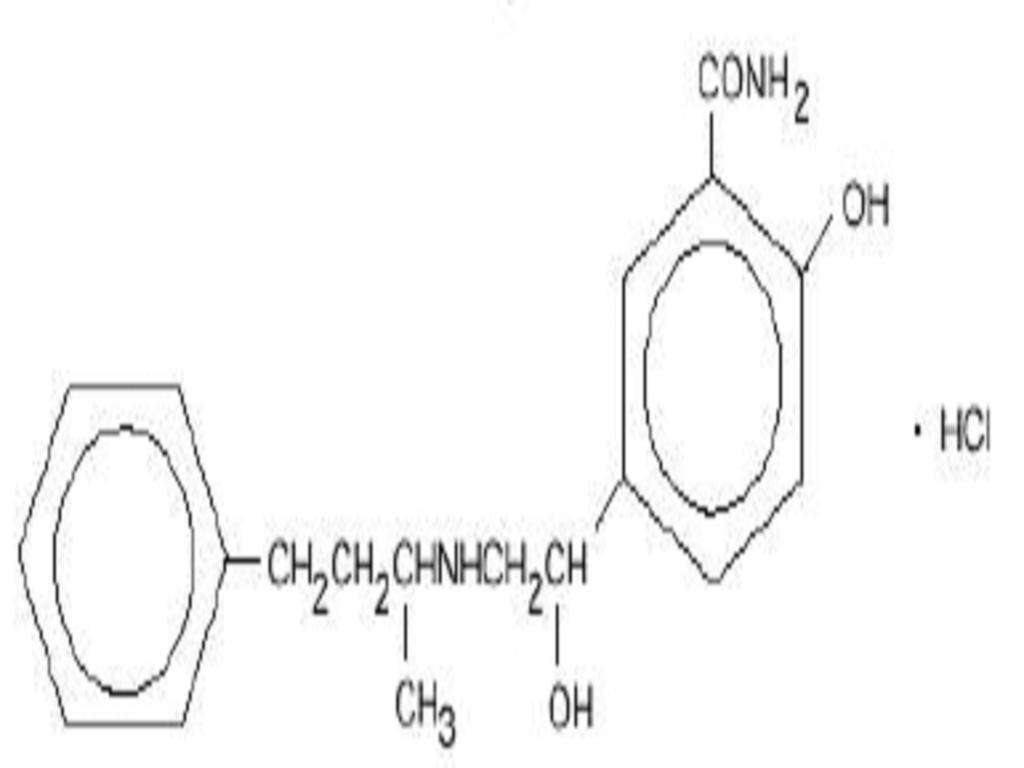
LABETALOL HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
PHARMACOKINETICS
Pharmacokinetics and MetabolismINDICATIONS & USAGE
LABETALOL HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Hepatic InjuryCardiac Failure
In Patients Without a History of Cardiac Failure
Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal
Nonallergic Bronchospasm (e.g., Chronic Bronchitis and Emphysema)
Pheochromocytoma
Diabetes Mellitus and Hypoglycemia
Major Surgery
PRECAUTIONS
GeneralImpaired Hepatic Function
Jaundice or Hepatic Dysfunction
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
Risk of Anaphylactic Reaction
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
Nonteratogenic Effects
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
LABETALOL HYDROCHLORIDE ADVERSE REACTIONS
Body as a Whole
Cardiovascular
Central and Peripheral Nervous Systems
Collagen Disorders
Eyes
Immunological System
Liver and Biliary System
Musculoskeletal System
Respiratory System
Skin and Appendages
Urinary System
Hypersensitivity
Potential Adverse Effects
Central Nervous System
Cardiovascular
Allergic
Hematologic
Gastrointestinal
Clinical Laboratory Tests
OVERDOSAGE
DOSAGE & ADMINISTRATION
Elderly Patients
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Labetalol HydrochlorideLabetalol Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!