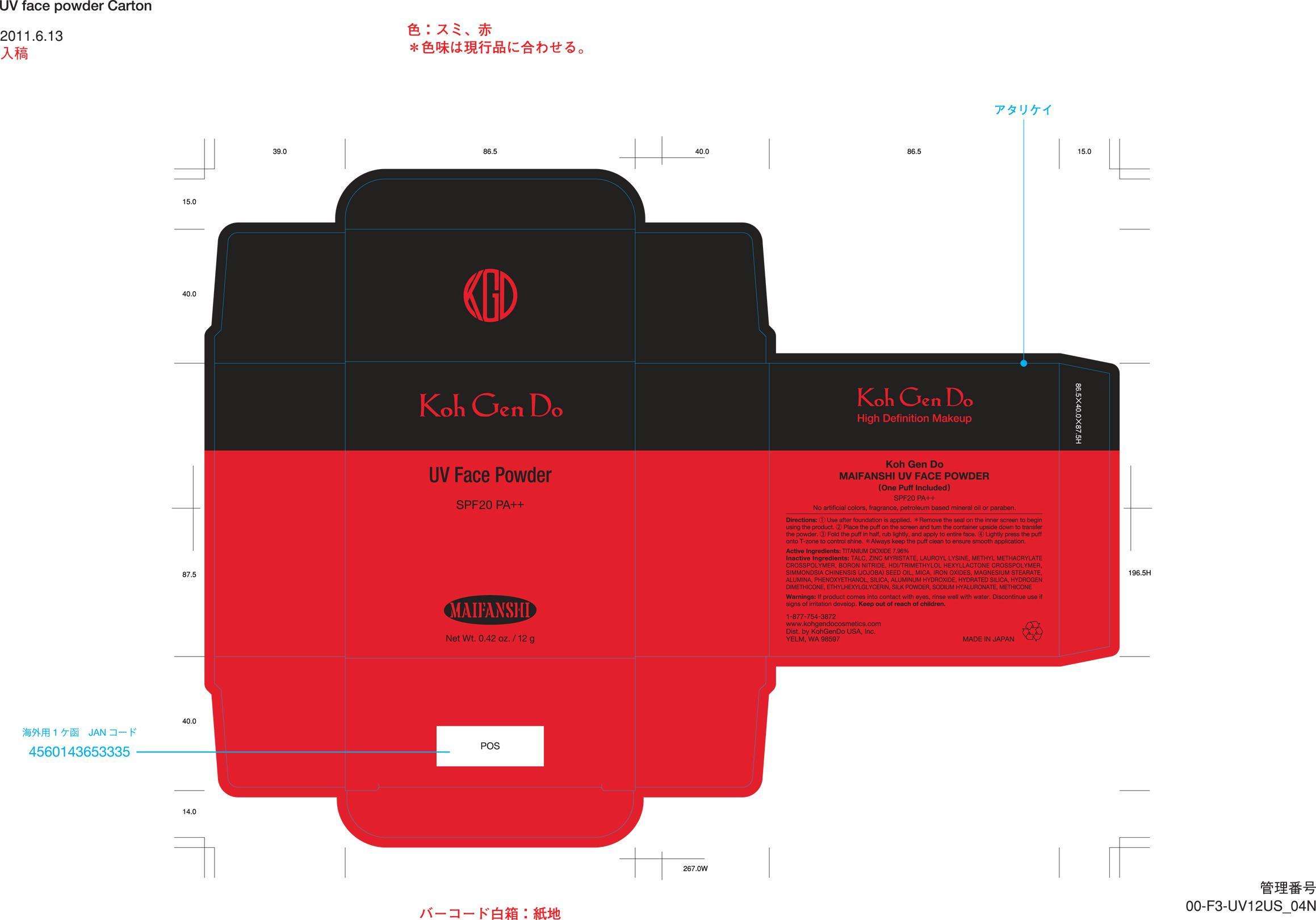
Koh Gen Do Maifanshi UV Face Powder
FULL PRESCRIBING INFORMATION
TITANIUM DIOXIDE 7.96%
If product comes into contact with eyes, rinse well with water. Discontinue use if signs of irritation develop. Keep out of reach of children.
1 Use after foundation is applied. *Remove the seal on the inner screen to begin using the product. 2 Place the puff on the screen and turn the container upside down to transfer the powder. 3 Fold the puff in half, rub lightly, and apply to entire face. 4 Lightly press the puff onto T-zone to control shine. *Always keep the puff clean to ensure smooth application.
(One Puff Included)
SPF20 PA++
No artificial colors, fragrance, petroleum based mineral oil or paraben.
1-877-754-3872
www.kohgendocosmetics.com
Dist. by KohGenDo USA, Inc. YELM, WA 98597
MADE IN JAPAN
TALC, ZINC MYRISTATE, LAUROYL LYSINE, BORON NITRIDE, SIMMONDSIA CHINENSIS (JOJOBA) SEED OIL, MICA, IRON OXIDES, MAGNESIUM STEARATE, ALUMINA, PHENOXYETHANOL, SILICA, ALUMINUM HYDROXIDE, HYDRATED SILICA, ETHYHEXYLGLYCERIN, SODIUM HYALURONATE, METHICONE
Koh Gen Do
UV Face Powder
SPF20 PA++
MAIFANSHI
Net Wt. 0.42 oz. / 12 g
Koh Gen Do
High Definition Makeup
Koh Gen Do
MAIFANSHI UV FACE POWDER
Koh Gen Do Maifanshi UV Face Powdertitanium dioxide POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||