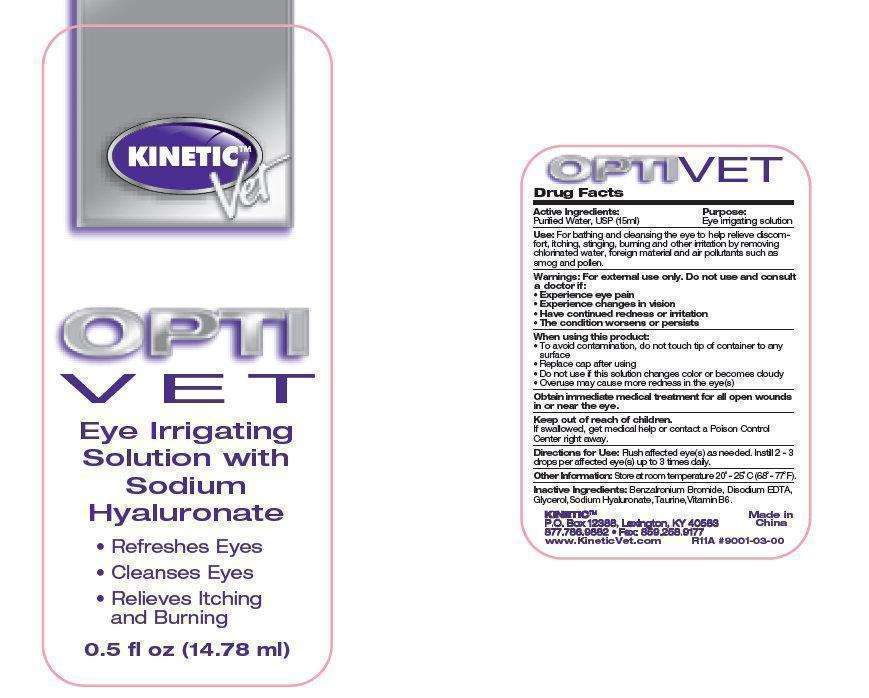
KINETIC VET OPTIVET Eye Irrigating
KINETIC VET OPTIVET Eye Irrigating Solution
FULL PRESCRIBING INFORMATION: CONTENTS*
- KINETIC VET OPTIVET Eye Irrigating Solution
- KINETIC VET OPTIVET Eye Irrigating Uses:
- Warnings:
- Direction for Use:
- Other Information:
- Inactive Ingredients:
- KINETIC VET OPTIVET Eye Irrigating Solution 14.78ml (51031-011-15)
FULL PRESCRIBING INFORMATION
KINETIC VET OPTIVET Eye Irrigating Solution
Active ingredient
Purified Water, USP (15ml)
Purpose:
Eye irrigating solution
Uses:
For bathing and cleansing the eye to help relieve discomfort, itching, stinging, burning and other irritation by removing chlorinated water, foreign material and air pollutants such as smog and pollen.
Warnings:
For external use only.
Do not use and consult a doctor if:
- Experience eye pain
- Experience changes in vision
- Have continued redness or irritation
- The condition worsens or persists
When using this product:
- To avoid contamination, do not touch tip of container to any surface
- Replace cap after using
- Do not use if this solution changes color or becomes cloudy
- Overuse may cause more redness in the eye(s)
Obtain immediate medical treatment for all open wounds in or near the eye.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Direction for Use:
Flush affected eye(s) as needed. Instill 2-3 drops per affected eye(s) up to 3 times daily.
Other Information:
Store at room temperature 200 - 250 C(680 - 770 F).
Inactive Ingredients:
Benzakonium Bromide, Disodium EDTA, Glycerol, Sodium Hyaluronate, Taurine, Vitamin B6.
KINETIC VET OPTIVET Eye Irrigating Solution 14.78ml (51031-011-15)
KINETIC VET OPTIVET Eye IrrigatingWATER SOLUTION/ DROPS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||