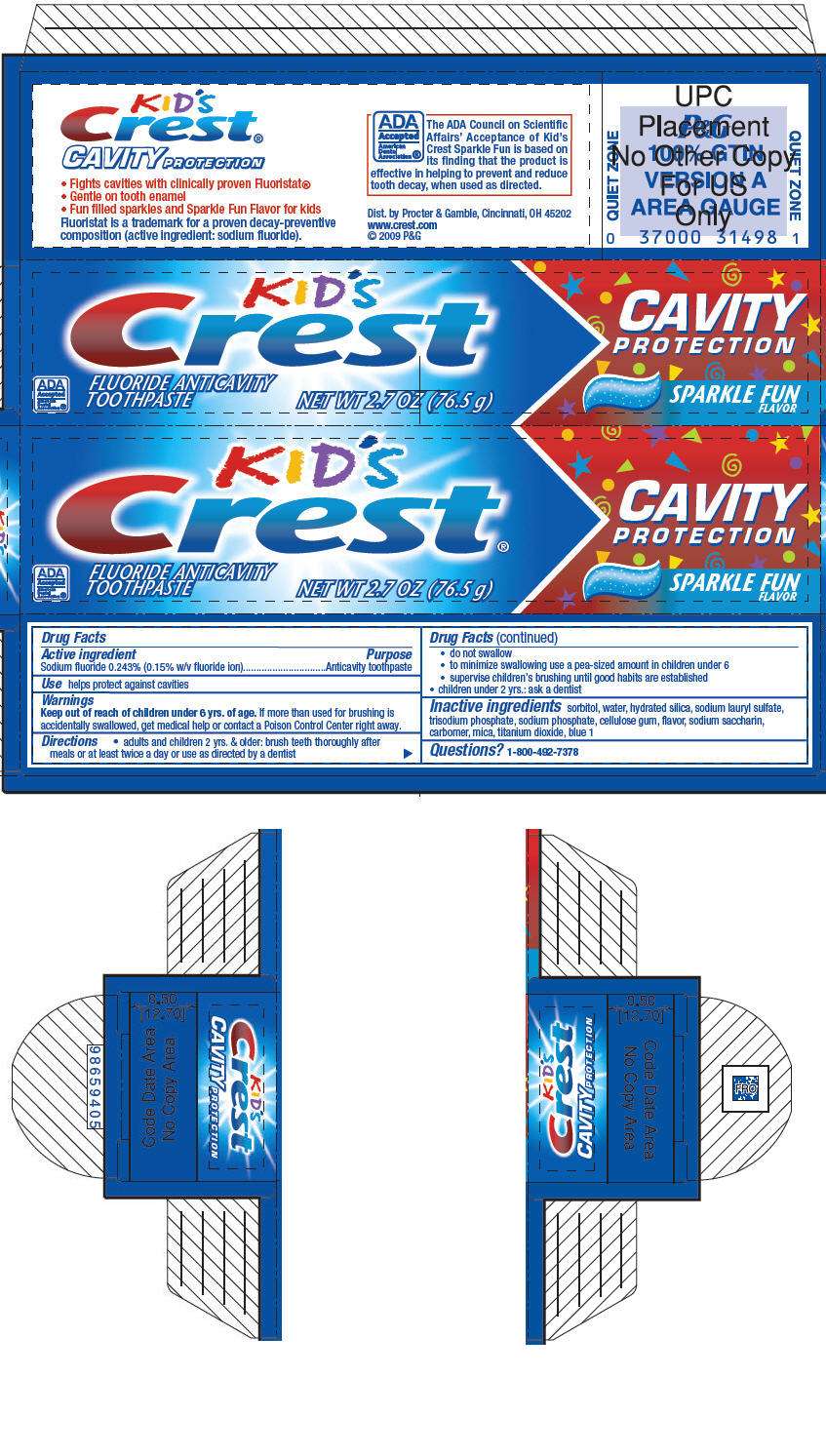
Kids Crest Cavity Protection
Procter & Gamble Manufacturing Company
Kid's Crest Cavity Protection
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 76.5 g Tube Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Sodium fluoride 0.243% (0.15% w/v fluoride ion)
Purpose
Anticavity toothpaste
Use
helps protect against cavities
Warnings
Keep out of reach of children under 6 yrs. of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 yrs. & older: brush teeth thoroughly after meals or at least twice a day or use as directed by a dentist
- do not swallow
- to minimize swallowing use a pea-sized amount in children under 6
- supervise children's brushing until good habits are established
- children under 2 yrs.: ask a dentist
Inactive ingredients
sorbitol, water, hydrated silica, sodium lauryl sulfate, trisodium phosphate, sodium phosphate, cellulose gum, flavor, sodium saccharin, carbomer, mica, titanium dioxide, blue 1
Questions?
1-800-492-7378
PRINCIPAL DISPLAY PANEL - 76.5 g Tube Carton
KID'S
Crest®
CAVITY
PROTECTION
ADA
Accepted
American
Dental
Association®
FLUORIDE ANTICAVITY
TOOTHPASTE
SPARKLE FUN
FLAVOR
NET WT 2.7 OZ (76.5 g)
Kids Crest Cavity ProtectionSodium Fluoride PASTE, DENTIFRICE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||