Ketodan
Medimetriks Pharmaceuticals, Inc.
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Ketodan™ Foam safely and effectively. See full prescribing information for Ketodan™ Foam. Ketodan™ (Ketoconazole Foam, 2%)For topical use only Initial U.S. Approval: 1981INDICATIONS AND USAGEKetodan™ Foam is indicated for topical treatment of seborrheic dermatitis in immunocompetent patients 12 years of age and older (1).Safety and efficacy of Ketodan™ Foam for treatment of fungal infections have not been established.DOSAGE AND ADMINISTRATION• Ketodan™ Foam should be applied to the affected area(s) twice daily for four weeks (2).• Ketodan™ Foam is not for ophthalmic, oral or intravaginal use (2).DOSAGE FORMS AND STRENGTHSKetodan™ Foam contains 2% ketoconazole in a thermolabile hydroethanolic foam in 100 g container (3).CONTRAINDICATIONSNoneWARNINGS AND PRECAUTIONS• Ketoconazole Foam, 2% may result in contact sensitization, including photoallergenicity (5.1, 6.2).• The contents of Ketoconazole Foam, 2% are flammable (5.2).Side EffectsThe most common adverse reactions observed in clinical studies (incidence >1%) were application site burning and application site reaction (6.1). To report SUSPECTED ADVERSE REACTIONS, contact Perrigo at 1-866-634-9120 or www.perrigo.com and FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONS
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 KETODAN INDICATIONS AND USAGE
- 2 KETODAN DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 KETODAN CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 KETODAN ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 11 KETODAN DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Ketodan™ Foam is indicated for the topical treatment of seborrheic dermatitis in immunocompetent patients 12 years of age and older. Safety and efficacy of Ketodan™ Foam for treatment of fungal infections have not been established.
2 DOSAGE AND ADMINISTRATION
Ketodan™ Foam should be applied to the affected area(s) twice daily for four weeks. Hold the container upright, and dispense Ketodan™ Foam into the cap of the can or other cool surface in an amount sufficient to cover the affected area(s). Dispensing directly onto hands is not recommended, as the foam will begin to melt immediately upon contact with warm skin. Pick up small amounts of Ketodan™ Foam with the fingertips, and gently massage into the affected area(s) until the foam disappears. For hair-bearing areas, part the hair, so that Ketodan™ Foam may be applied directly to the skin (rather than on the hair).
Avoid contact with the eyes and other mucous membranes. Ketodan™ Foam is not for ophthalmic, oral or intravaginal use.
3 DOSAGE FORMS AND STRENGTHS
Ketodan™ Foam contains 2% ketoconazole in a thermolabile hydroethanolic foam, and is provided in a 100 g aluminum container.
4 CONTRAINDICATIONS
None
5 WARNINGS AND PRECAUTIONS
5.1 Contact Sensitization
Ketodan™ Foam may result in contact sensitization, including photoallergenicity. [See Adverse Reactions (6.2) ]
5.2 Flammable Contents
The contents of Ketodan™ Foam include alcohol and propane/butane, which are flammable. Avoid fire, flame and/or smoking during and immediately following application. Do not puncture and/or incinerate the containers. Do not expose containers to heat and/or store at temperatures above 120°F (49°C).
5.3 Systemic Effects
Hepatitis has been seen with orally administered ketoconazole (1:10,000 reported incidence). Lowered testosterone and ACTH–induced corticosteroid serum levels have been seen with high doses of orally administered ketoconazole. These effects have not been seen with topical ketoconazole.
6 ADVERSE REACTIONS
6.1 Side Effects in Clinical Trials
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse reactions that appear to be related to drug use and for approximating rates.
The safety data presented in Table 1 (below) reflect exposure to ketoconazole foam, 2% in 672 subjects, 12 years and older with seborrheic dermatitis. Subjects applied ketoconazole foam, 2% or vehicle foam twice daily for 4 weeks to affected areas on the face, scalp, and/or chest. Adverse reactions occurring in > 1% of subjects are presented in Table 1.
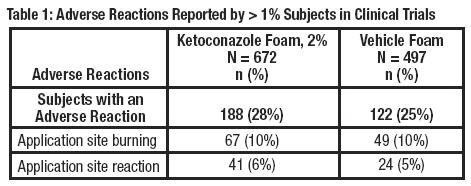
Application site reactions that were reported in ≤1% of subjects were dryness, erythema, irritation, paresthesia, pruritus, rash and warmth.
6.2 Dermal Safety Studies
In a photoallergenicity study, 9 of 53 subjects (17%) had reactions during the challenge period at both the irradiated and non-irradiated sites treated with ketoconazole foam, 2%. Ketoconazole foam, 2% may cause contact sensitization.
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C
Ketoconazole has been shown to be teratogenic (syndactylia and oligodactylia) in the rat when given orally in the diet at 80 mg/kg/day (4.8 times the maximum expected human topical dose based on a mg/m2 comparison, assuming 100% absorption from 8 g of foam). However, these effects may be partly related to maternal toxicity, which was also observed at this dose level. [See Pharmacokinetics (12.3)]
No reproductive studies in animals have been performed with ketoconazole foam, 2%.
There are no adequate and well-controlled studies of ketoconazole foam, 2% in pregnant women. Ketoconazole foam, 2% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
It is not known whether ketoconazole foam, 2% administered topically could result in sufficient systemic absorption to produce detectable quantities in breast milk. Because many drugs are excreted in human milk, caution should be exercised when ketoconazole foam, 2% is administered to women who are breastfeeding.
8.4 Pediatric Use
The safety and effectiveness of ketoconazole foam, 2% in pediatric patients less than 12 years of age have not been established.
Of the 672 subjects treated with ketoconazole foam, 2% in the clinical trials, 44 (7%) were from 12 to 17 years of age. [See Clinical Studies (14)]
8.5 Geriatric Use
Of the 672 subjects treated with ketoconazole foam, 2% in the clinical trials, 107 (16%) were 65 years and over.
11 DESCRIPTION
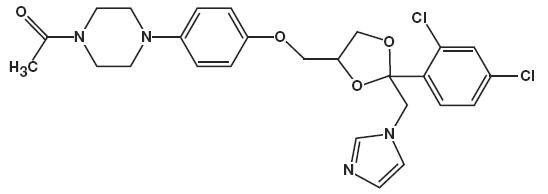
Ketodan™ Foam contains 2% ketoconazole USP, an antifungal agent, in a thermolabile hydroethanolic foam for topical application.
The chemical name for ketoconazole is piperazine, 1-acetyl-4-[4-[[2-(2,4-dichlorophenyl) -2-(1H-imidazol-1-ylmethyl)-1, 3-dioxolan-4-yl]methoxy]phenyl]-, cis- with the molecular formula C26H28CI2N4O4 and a molecular weight of 531.43.
The following is the chemical structure:
Ketodan™ Foam contains 20 mg ketoconazole USP per gram in a thermolabile hydroethanolic foam vehicle consisting of cetyl alcohol NF, citric acid USP, ethanol (denatured with tert-butyl alcohol and brucine sulfate) 58%, polysorbate 60 NF, potassium citrate USP, propylene glycol USP, purified water USP, and stearyl alcohol NF pressurized with a hydrocarbon (propane/butane) propellant.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of ketoconazole in the treatment of seborrheic dermatitis is not known.
12.2 Pharmacodynamics
The pharmacodynamics of ketoconazole foam, 2% has not been established.
12.3 Pharmacokinetics
In a bioavailability study, 12 subjects with moderate to severe seborrheic dermatitis applied 3 g of ketoconazole foam, 2% twice daily for 4 weeks. Circulating plasma levels of ketoconazole were < 6 ng/mL for a majority of subjects (75%), with a maximum level of 11 ng/mL observed in one subject.
12.4 Microbiology
Ketoconazole is an antifungal agent which inhibits the in vitro synthesis of ergosterol, a key sterol in the cell membrane of Malassezia furfur. The clinical significance of antifungal activity in the treatment of seborrheic dermatitis is not known.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic or photo-carcinogenic potential of ketoconazole foam, 2%.
In oral carcinogenicity studies in mice (18-months) and rats (24-months) at dose levels of 5, 20 and 80 mg/kg/day ketoconazole was not carcinogenic. The high dose in these studies was approximately 2.4 to 4.8 times the expected topical dose in humans based on a mg/m2 comparison. In a bacterial reverse mutation assay, ketoconazole did not express any mutagenic potential. In three in vivo assays (sister chromatid exchange in humans, dominant lethal and micronucleus tests in mice), ketoconazole did not exhibit any genotoxic potential.
At oral dose levels of 75 mg/kg/day (4.5 times the expected topical human dose in mg/m2), ketoconazole impaired reproductive performance and fertility when administered to male rats (increased abnormal sperm, decreased sperm mobility and decreased pregnancy in mated females).
14 CLINICAL STUDIES
The safety and efficacy of ketoconazole foam, 2% were evaluated in a randomized, double-blind, vehicle-controlled study in subjects 12 years and older with mild to severe seborrheic dermatitis. In the study, 427 subjects received ketoconazole foam, 2% and 420 subjects received vehicle foam. Subjects applied ketoconazole foam, 2% or vehicle foam twice daily for 4 weeks to affected areas on the face, scalp, and/or chest. The overall disease severity in terms of erythema, scaling, and induration was assessed at Baseline and week 4 on a 5-point Investigator's Static Global Assessment (ISGA) scale.
Treatment success was defined as achieving a Week 4 (end of treatment) ISGA score of 0 (clear) or 1 (majority of lesions have individual scores for scaling, erythema, and induration that averages 1 [minimal or faint]) and at least two grades of improvement from baseline. The results are presented in Table 2. The database was not large enough to assess whether there were differences in effects in age, gender, or race subgroups.

16 HOW SUPPLIED/STORAGE AND HANDLING
Ketodan™ Foam is supplied in 100 g (NDC 43538-530-10) aluminum container.
Store at 20-25°C (68-77°F) [See USP Controlled Room Temperature]. Do not store under refrigerated conditions. Do not expose containers to heat, and/or store at temperatures above 120°F (49°C). Do not store in direct sunlight.
Contents are flammable.
Contents under pressure. Do not puncture and/or incinerate container.
Keep out of reach of children.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling. (17.3 )
17.1 Instructions for Use
• Avoid fire, flame and/or smoking during and immediately following application.
• Do not apply Ketodan™ Foam directly to hands. Dispense onto a cool surface, and apply to the affected areas using the fingertips.
17.2 Local Reactions
• Ketodan™ Foam may cause skin irritation (application site burning and/or reactions)
• Ketodan™ Foam may cause contact sensitization.
• As with any topical medication, patients should wash their hands after application.
• Inform a physician if the area of application shows signs of increased irritation and report any signs of adverse reactions.
17.3 Patient Package Insert
- See below -
Medimetriks Pharmaceuticals, Inc.
363 Route 46 West, Fairfield, NJ 07004
www.medimetriks.com
PATIENT INFORMATION
Medimetriks Pharmaceuticals, Inc.
363 Route 46 West, Fairfield, NJ 07004
www.medimetriks.com
Ketodan™ (Ketoconazole Foam, 2%)
IMPORTANT: For skin use only. Do not use in the eyes, mouth or vagina.
Read the Patient Information that comes with Ketodan™ Foam before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition or treatment.
What is Ketodan™ Foam?
Ketodan™ Foam is used on the skin (topical) to treat a skin condition called seborrheic dermatitis in patients 12 years and older. Seborrheic dermatitis can cause areas of flaky skin (scales) on the scalp, face, ears, chest or upper back.
Ketoconazole Foam, 2% has not been studied in children less than 12 years old.
What should I tell my doctor before using Ketodan™ Foam?
For female patients, tell your doctor if you:
• are pregnant or become pregnant. It is not known if Ketodan™ Foam can harm a fetus (unborn baby).
• breastfeeding. It is not known if Ketodan™ Foam passes into breast milk.
How should I use Ketodan™ Foam?
• Apply Ketodan™ Foam exactly as prescribed. Ketodan™ Foam is usually applied to the affected skin areas two times a day (once in the morning and once at night) for 4 weeks. Talk to your doctor if your skin does not improve after 4 weeks of treatment with Ketoconazole Foam, 2%.
• Keep the Ketodan™ Foam can away from and do not spray it near fire, open flame, or direct heat. Ketodan™ Foam is flammable. Never throw the Ketodan™ Foam can into a fire, even if the can is empty.
Instructions for applying Ketodan™ Foam
|
1. Hold the can at an upright angle. 2. Push the button to spray Ketodan™ Foam directly into the cap of the can or other cool surface. Spray only the amount of Ketodan™ Foam that you will need to cover your affected skin. Do not spray Ketodan™ Foam directly onto your affected skin or your hands because the foam will begin to melt right away when it touches your skin. 3. If your fingers are warm, rinse them in cold water first. Be sure to dry them well before handling the Ketodan™ Foam. If the Ketodan™ Foam can seems warm or the foam seems runny, place the can under cool running water for a few minutes. 4. Using your fingertips, gently massage Ketodan™ Foam into the affected areas until the foam disappears. 5. If you are treating skin areas with hair such as your scalp, move any hair away so that the foam can be applied to the affected skin. 6. Do not get Ketodan™ Foam in your eyes, mouth or vagina. If any Ketodan™ Foam gets in your eyes, mouth or vagina, rinse areas well with water. 7. Wash your hands well after applying Ketodan™ Foam. |

|
|
|
|

|
What are the possible side effects of Ketodan™ Foam?
The most common side effects of Ketodan™ Foam are reaction or burning on treated skin areas. Tell your doctor if you have any reaction on your treated skin such as redness, itching, or a rash. These are not all the side effects of Ketodan™ Foam. Ask your doctor or pharmacist for more information.
How should I store Ketodan™ Foam?
• Ketodan™ Foam is flammable.
• Do not spray Ketodan™ Foam near fire or direct heat. Never throw the can into a fire, even if the can is empty.
• Store the can of Ketodan™ Foam at room temperature, 20-25°C (68-77°F) [See USP Controlled Room Temperature]. Do not place the Ketodan™ Foam can in the refrigerator or freezer.
• Keep the Ketodan™ Foam can away from all sources of fire and heat. Do not leave the Ketodan™ Foam can in direct sunlight.
• Do not smoke while holding the Ketodan™ Foam can or while spraying or applying the foam.
• Do not pierce or burn the Ketodan™ Foam can.
• Keep Ketodan™ Foam and all medicines out of the reach of children.
General information about Ketodan™ Foam
Medicines are sometimes prescribed for conditions that are not mentioned in Patient Information leaflets. Do not use Ketodan™ Foam for any other condition for which it was not prescribed. Do not give Ketodan™ Foam to other people, even if they have the same condition that you have. It may harm them.
This leaflet summarizes the most important information about Ketodan™ Foam. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Ketodan™ Foam that is written for health professionals.
What are the ingredients in Ketodan™ Foam?
Active ingredient: ketoconazole, USP
Inactive Ingredients: cetyl alcohol NF, citric acid USP, ethanol (denatured with tert-butyl alcohol and brucine sulfate) 58%, polysorbate 60 NF, potassium citrate USP, propylene glycol USP, purified water USP, and stearyl alcohol NF pressurized with a hydrocarbon (propane/butane) propellant.
This Patient Information leaflet has been approved by the U.S. Food and Drug Administration.
The Patient Information leaflet was last revised: August 2010
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Rx Only
Made in Israel
Medimetriks Pharmaceuticals, Inc.
363 Route 46 West, Fairfield, NJ 07004
www.medimetriks.com
Manufactured by Perrigo Yeruham 80500, Israel
Iss:11/11
Distributed By
Perrigo®
Allegan, MI 49010 • www.perrigo.com
Rev. 08/2010
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC 43538-531-10
Rx Only
Ketodan
™
Ketoconazole
Foam, 2% KIT
For Topical Use Only.
Not For Ophthalmic, Oral, or Intravaginal Use.
CONTENTS:
1 - Ketodan™ (Ketoconazole Foam, 2%) - Net wt. 3.53 oz. (100 g)
1 - Rehyla™ Hair & Body Cleanser - Net wt. 16 oz. (454 g)
MEDIMETRIKS
PHARMACEUTICALS, INC.

PRINCIPAL DISPLAY PANEL - 100 g Can Carton
NDC 43538-530-10
Rx Only
Ketodan™
Ketoconazole Foam, 2%
For Topical Use Only.
Not For Ophthalmic, Oral,
or Intravaginal Use.
Net wt. 3.53 oz.
(100 g)
MEDIMETRIKS
PHARMACEUTICALS, INC.

Ketodanketoconazole AEROSOL, FOAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ketodanketoconazole KIT
| ||||||||||||||||||||||||||||||||||||||||