Kepivance
Swedish Orphan Biovitrum AB (publ)
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Kepivance safely and effectively. See full prescribing information for KEPIVANCE.Kepivance® (palifermin) For injection, for intravenous useInitial U.S. Approval: 2004 INDICATIONS AND USAGE Kepivance is a mucocutaneous epithelial human growth factor indicated to decrease the incidence and duration of severe oral mucositis in patients with hematologic malignancies receiving myelotoxic therapy requiring hematopoietic stem cell support. Kepivance is indicated as supportive care for preparative regimens predicted to result in ≥ WHO Grade 3 mucositis in the majority of patients. (1.1) Limitations of Use The safety and efficacy of Kepivance have not been established in patients with non-hematologic malignancies (1.2, 5.1) Kepivance is not recommended for use with melphalan 200 mg/m2 as a conditioning regimen (14.2). DOSAGE AND ADMINISTRATIONAdminister as an intravenous bolus injection at a dose of 60 mcg/kg/day for 3 consecutive days before and 3 consecutive days after myelotoxic therapy for a total of 6 doses (2.1) Administer the first 3 doses prior to myelotoxic therapy with the third dose 24 to 48 hours before myelotoxic therapy (2.1) Administer the last 3 doses after myelotoxic therapy is complete with the first of these doses on the day of hematopoietic stem cell infusion after the infusion is completed, and more than 4 days after the most recent administration of Kepivance (2.1) DOSAGE FORMS AND STRENGTHS6.25 mg lyophilized powder in single-use vials (3) CONTRAINDICATIONSNone WARNINGS AND PRECAUTIONS Potential for stimulation of tumor growth — Kepivance is not indicated for non-hematologic tumors. The effects of Kepivance on stimulation of keratinocyte growth factor (KGF) receptor-expressing, non-hematopoietic tumors in patients are not known (1, 5.1) Side EffectsMost common adverse reactions (incidence ≥20% and 5%≥placebo) are rash, fever, elevated serum amylase (Grade 3/4), pruritus, erythema, and edema (6) To report SUSPECTED ADVERSE REACTIONS, contact Swedish Orphan Biovitrum at 1-866-773-5274 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Heparin may increase systemic exposure (7) Myelotoxic chemotherapy (7, 14) USE IN SPECIFIC POPULATIONS Pregnancy: Based on animal data, may cause fetal harm (8.1) Nursing Mothers: Consider discontinuation of drug or nursing, taking into account the importance of drug to mother. (8.3)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 KEPIVANCE INDICATIONS AND USAGE
- 2 KEPIVANCE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 KEPIVANCE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 KEPIVANCE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 KEPIVANCE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Indications
Kepivance is a mucocutaneous epithelial human growth factor indicated to decrease the incidence and duration of severe oral mucositis in patients with hematologic malignancies receiving myelotoxic therapy requiring hematopoietic stem cell support. Kepivance is indicated as supportive care for preparative regimens predicted to result in ≥ WHO Grade 3 mucositis in the majority of patients.
1.2 Limitations of Use
The safety and efficacy of Kepivance have not been established in patients with non-hematologic malignancies [see Warnings and Precautions (5.1 )].
Kepivance is not recommended for use with melphalan 200 mg/m2 as a conditioning regimen [See Clinical Studies (14.2)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage Regimen
The recommended dose of Kepivance is 60 mcg/kg/day, administered as an intravenous bolus injection for 3 consecutive days before and 3 consecutive days after myelotoxic therapy, for a total of 6 doses.
Administer the first 3 doses prior to myelotoxic therapy. Administer the third dose 24 to 48 hours prior to beginning myelotoxic therapy [see Drug Interactions (7)].
Administer the last 3 doses after myelotoxic therapy is complete; Administer the first of these doses on the day of hematopoietic stem cell infusion after the infusion is completed, and more than 4 days after the most recent administration of Kepivance [see Drug Interactions (7)].
2.2 Preparation and Administration
Preparation
Prepare the solution for infusion, using aseptic technique, as follows:
- Reconstitute Kepivance lyophilized powder with Sterile Water for Injection, USP (not supplied) by slowly injecting 1.2 mL of Sterile Water for Injection, USP to yield a final concentration of 5 mg/mL.
- Swirl the contents gently during dissolution. Do not shake or vigorously agitate the vial. Dissolution of Kepivance can take up to 3 minutes.
- Visually inspect the solution for discoloration and particulate matter before administration. The reconstituted solution should be clear and colorless. Do not administer Kepivance if discoloration or particulates are observed. Do not filter the reconstituted solution during preparation or administration. Do not freeze the reconstituted solution. Protect from light.
Administration
- Administer Kepivance by intravenous bolus injection. If heparin is used to maintain an intravenous line, rinse the line with saline prior to and after Kepivance administration [see Drug Interactions (7)].
- The reconstituted solution contains no preservatives and is intended for single use only. Discard any unused portion.
- Following reconstitution, it is recommended that the product be used immediately. If not used immediately, the reconstituted solution of Kepivance may be stored refrigerated in its carton at 2° to 8°C (36° to 46°F) for up to 24 hours.
- Prior to injection, allow Kepivance to reach room temperature for a maximum of 1 hour protected from light. Discard Kepivance left at room temperature for more than 1 hour.
3 DOSAGE FORMS AND STRENGTHS
6.25 mg lyophilized powder in single-use vials.
4 CONTRAINDICATIONS
None
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Stimulation of Tumor Growth
The safety and efficacy of Kepivance have not been established in patients with non-hematologic malignancies. The effects of Kepivance on stimulation of KGF receptor-expressing, non-hematopoietic tumors in patients are not known. Kepivance has been shown to enhance the growth of human epithelial tumor cell lines in vitro and to increase the rate of tumor cell line growth in a human carcinoma xenograft model [see Clinical Pharmacology (12.1)].
6 ADVERSE REACTIONS
The most common adverse reactions attributed to Kepivance were skin toxicities (rash, erythema, edema, pruritus), oral toxicities (dysesthesia, tongue discoloration, tongue thickening, alteration of taste), pain, arthralgias, and dysesthesia. The median time to onset of cutaneous toxicity was 6 days following the first of 3 consecutive daily doses of Kepivance, with a median duration of 5 days. In patients receiving Kepivance, dysesthesia (including hyperesthesia, hypoesthesia, and paresthesia) was usually localized to the perioral region, whereas in patients receiving placebo dysesthesias were more likely to occur in extremities.
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described in Table 1 and the discussion below reflect exposure to Kepivance in 409 patients with hematologic malignancies who were enrolled in 3 randomized, placebo-controlled clinical trials and a pharmacokinetic study. Patients received Kepivance either before, or before and after, regimens of myelotoxic chemotherapy, with or without total body irradiation (TBI), followed by hematopoietic stem cell support. Kepivance was administered in daily doses ranging from 5 to 80 mcg/kg/day. The total dose of Kepivance ranged from 15 to 480 mcg/kg with a median of 360 mcg/kg. The population had a median age of 48 years (range: 41 to 60 years), 62% were male and 83% were White with 7.4 % Black and 6.2 % Hispanic. Non Hodgkin's lymphoma (NHL) was the most common malignancy followed by Hodgkin's disease, multiple myeloma, and leukemia.
The most common serious adverse reaction attributed to Kepivance was skin rash, reported in less than 1% (3/409) of patients treated. Grade 3 skin rashes occurred in 3% of patients (9/409) receiving Kepivance and 2% (5/241) receiving placebo.
|
BODY SYSTEM
Adverse Event |
Kepivance
(n = 409) % |
Placebo
(n = 241) % |
| BODY AS A WHOLE | ||
| Edema | 28 | 21 |
| Pain | 16 | 11 |
| Fever | 39 | 34 |
| GASTROINTESTINAL | ||
| Mouth/Tongue Thickness or Discoloration | 17 | 8 |
| MUSCULOSKELETAL | ||
| Arthralgia | 10 | 5 |
| SKIN AND APPENDAGES | ||
| Rash | 62 | 50 |
| Pruritus | 35 | 24 |
| Erythema | 32 | 22 |
| SPECIAL SENSES | ||
| Taste Altered | 16 | 8 |
| CENTRAL NERVOUS SYSTEM / PERIPHERAL NERVOUS SYSTEM | ||
| Dysesthesia – Hyperesthesia / hypoesthesia/ paresthesia | 12 | 7 |
| METABOLIC | ||
| Elevated serum lipase |
||
| All grades | 28 | 23 |
| Grade 3 and 4 | 11 | 5 |
| Elevated serum amylase | ||
| All grades | 62 | 54 |
| Grade 3 and 4 | 38 | 31 |
Cataracts: In a postmarketing safety study, the incidence of cataracts was numerically higher among patients receiving Kepivance than in the control population. (See 14 CLINICAL STUDIES).
Laboratory Test Findings: Reversible elevations in serum lipase and amylase, which did not require treatment, were reported in 28% and 62% of patients receiving Kepivance and 23% and 54%of patients receiving placebo. In general, peak increases were observed during the period of cytotoxic therapy and returned to baseline by the day of hematopoietic stem cell infusion. Amylase was mainly salivary in origin.
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The clinical significance of antibodies to Kepivance is unknown but may include decreased activity and/or cross reactivity with other members of the FGF family of growth factors.
In clinical trials, serum samples from patients treated with Kepivance were tested for antibodies to Kepivance using an electrochemiluminescence-based binding assay. Twelve of 645 patients (2%) tested positive; none had evidence of neutralizing activity in a cell-based assay.
The incidence of antibody positivity is highly dependent on the specific assay and its sensitivity. Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications and underlying disease. For these reasons, comparison of the incidence of antibodies to Kepivance with the incidence of antibodies to other products may be misleading.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Kepivance in the stem cell transplant setting. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Vaginal edema and erythema;
- Palmar-plantar Erythrodysaesthesia Syndrome (also known as “hand-foot syndrome”)
7 DRUG INTERACTIONS
In vitro and in vivo data showed that palifermin interacts with unfractionated as well as low molecular weight heparins. Heparin co-administration resulted in a 5-fold increase in palifermin systemic exposure. Avoid co-administration of palifermin with heparin. If heparin is used to maintain an intravenous line, rinse the line with saline prior to and after Kepivance administration [see Clinical Pharmacology (12.3)].
Do not administer Kepivance within 24 hours before, during infusion of, or within 24 hours after administration of myelotoxic chemotherapy [see Dosage and Administration (2.1) and Clinical Studies (14)]. In a clinical trial, administration of Kepivance within 24 hours of chemotherapy resulted in increased severity and duration of oral mucositis.
8 USE IN SPECIFIC POPULATIONS
No gender-related differences were observed in the pharmacokinetics of Kepivance at doses ≤ 60 mcg/kg.
8.1 Pregnancy
Pregnancy Category C: There are no adequate and well-controlled studies of Kepivance in pregnant woman. Palifermin is embryotoxic in rabbits and rats. In reproductive toxicology studies, increased post-implantation loss and decrease in fetal body weight were observed in both rabbit (2.5 times the maximum recommended human dose [MRHD], adjusted for body weight) and rat (8 times the MRHD, on a mcg/kg basis) [see Nonclinical Toxicology (13.3)]. Kepivance should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
8.3 Nursing Mothers
It is not known whether Kepivance is secreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from Kepivance, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Information on the dosing and safety of Kepivance in the pediatric population is limited. However, use of Kepivance in pediatric patients ages 1 to 16 years is supported by evidence from adequate and well-controlled studies of Kepivance in adults and a phase 1 study that included 27 pediatric patients with acute leukemia undergoing hematopoietic stem cell transplant. Three age groups were studied: ages 1 to 2 (n=9), ages 3 to 11 (n=9), and ages 12 to 16 (n=9); 56% were male, 26% were Caucasian, 63% Hispanic; 81% ALL, 19% AML. The patients received high-dose cytotoxic therapy consisting of fractionated total body irradiation (TBI) (12 Gy total dose), high dose etoposide (1500 mg/m2), and high dose cyclophosphamide (120 mg/kg) followed by allogeneic hematopoietic stem cell support. The dose intensity of this preparative regimen is comparable to the dose intensity of the Study 1 preparative regimen. See Clinical Studies [14.1]. Kepivance was administered as a daily intravenous injection for 3 consecutive days prior to initiation of cytotoxic therapy and for 3 consecutive days following infusion of hematopoietic stem cells. Three dose levels, 40, 60, and 80 mcg/kg/dose, were evaluated. There was no dose limiting toxicity identified at any dose level. Adverse events were similar to those reported in adult studies. The incidence of palifermin related adverse events was highest in the 80 μg/kg cohort. The overall incidence of WHO grade 3 and 4 oral mucositis was 10/27 (37%).
The pharmacokinetics of Kepivance was evaluated in the phase 1 study. Age (1 to 16 years) did not affect the pharmacokinetics of palifermin over the dose range (40 to 80 mcg/kg). Palifermin concentrations declined in the first 30 minutes after dosing. An increase in palifermin concentrations occurred at around 2 to 4 hours post-dose for some subjects, which was followed by a second, slow decline phase. A similar trend has been observed in adult patients. The mean half-life range was 2.6 to 5.6 hours in pediatric patients following the first 60 mcg/kg dose of Kepivance. No accumulation was observed following 3 consecutive doses of Kepivance. Palifermin exposure did not increase linearly with increasing doses. The first dose AUC0-inf (mean) of Kepivance 60 mcg/kg/day in adult patients (18 to 63 years) was 38.2 ng*hr/mL compared to 46.1 ng*hr/mL (range of means: 22.8 to 81.6) for pediatric patients (1 to 16 years). The mean clearance was 1730 mL/hr/kg for adults and 2481 mL/hr/kg (range of means: 1700 to 3460) in pediatric patients.
8.5 Geriatric Use
Clinical studies of Kepivance did not include sufficient numbers of subjects aged 65 years and older to determine whether they responded differently from younger subjects. [see Clinical Pharmacology (12.3)].
8.6 Patients with Renal Impairment
No dose adjustment is recommended for patients with renal impairment [see Clinical Pharmacology (12.3)].
8.7 Patients with Hepatic Impairment
The pharmacokinetic profile in patients with hepatic insufficiency has not been assessed.
10 OVERDOSAGE
No data are available regarding overdosage with Kepivance.
11 DESCRIPTION
Kepivance (palifermin) is a truncated human KGF produced by recombinant DNA technology in E coli. Kepivance is a water soluble, 140 amino acid protein with a molecular weight of 16.3 kilodaltons. It differs from endogenous human KGF in that the first 23 N terminal amino acids have been deleted to improve protein stability.
Kepivance is supplied as a sterile, white, preservative-free, lyophilized powder for intravenous injection after reconstitution with 1.2 mL of Sterile Water for Injection, USP. Reconstitution yields a clear, colorless solution of Kepivance (5 mg/mL) with a pH of 6.5. Each single use vial of Kepivance contains palifermin (6.25 mg),with L histidine (1.94 mg), mannitol (50 mg), polysorbate 20 (0.13 mg or 0.01% w/v), and sucrose (25 mg).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
KGF is an endogenous protein in the fibroblast growth factor (FGF) family that binds to the KGF receptor. Binding of KGF to its receptor has been reported to result in proliferation, differentiation, and migration of epithelial cells. The KGF receptor, one of four receptors in the FGF family, has been reported to be present on epithelial cells in many tissues examined including the tongue, buccal mucosa, esophagus, stomach, intestine, salivary gland, lung, liver, pancreas, kidney, bladder, mammary gland, skin (hair follicles and sebaceous gland), and the lens of the eye. The KGF receptor has been reported to not be present on cells of the hematopoietic lineage. Endogenous KGF is produced by mesenchymal cells and is upregulated in response to epithelial tissue injury.
In mice and rats, Kepivance enhanced proliferation of epithelial cells (as measured by Ki67 immunohistochemical staining and BrDU uptake) and demonstrated an increase in tissue thickness of the tongue, buccal mucosa, and gastrointestinal tract. Kepivance has been studied in murine models of chemotherapy and radiation-induced gastrointestinal injury. In such models, administration of Kepivance prior to and/or after the cytotoxic insult improved survival and reduced weight loss compared to control animals.
Kepivance has been shown to enhance the growth of human epithelial tumor cell lines in vitro at concentrations ≥ 10 mcg/mL (> 15-fold higher than average therapeutic concentrations in humans). In nude mouse xenograft models, three consecutive daily treatments of Kepivance at doses of 1,500 and 4,000 mcg/kg (25- and 67-fold higher than the recommended human dose, respectively) repeated weekly for 4 to 6 weeks were associated with a dose-dependent increase in the growth rate of 1 of 7 KGF receptor-expressing human tumor cell lines.
12.2 Pharmacodynamics
Epithelial cell proliferation was assessed by Ki67 immunohistochemical staining in healthy subjects. A 3-fold or greater increase in Ki67 staining was observed in buccal biopsies from 3 of 6 healthy subjects given Kepivance at 40 mcg/kg/day intravenously for 3 days, when measured 24 hours after the third dose. Dose-dependent epithelial cell proliferation was observed in healthy subjects given single intravenous doses of 120 to 250 mcg/kg 48 hours post-dosing.
12.3 Pharmacokinetics
The pharmacokinetics of Kepivance were studied in healthy subjects and patients with hematologic malignancies. After single intravenous doses of 20 to 250 mcg/kg in healthy subjects and 60 mcg/kg in cancer patients, Kepivance concentrations declined over 95% in the first 30 minutes post-dose. A slight increase or plateau in concentration occurred at approximately 1 to 4 hours, followed by a terminal decline phase. Kepivance exhibited linear pharmacokinetics with extravascular distribution. In cancer patients compared with healthy subjects, after a 60 mcg/kg single dose of Kepivance the average total body clearance (CL) was 2- to 4-fold higher, and volume of distribution at steady state (Vss) was 2-fold higher. The elimination half-life was similar between healthy subjects and cancer patients (average 4.5 hours with a range of 3.3 to 5.7 hours). No accumulation of Kepivance occurred after 3 consecutive daily doses of 20 and 40 mcg/kg in healthy subjects or 60 mcg/kg in cancer patients. Age (1 to 16 years) did not affect the pharmacokinetics of palifermin over the dose range of 40 to 80 mcg/kg [see Use in Specific Populations (8.4)].
Drug Interactions
Co-administration with Heparin
The potential pharmacokinetic interaction between palifermin and heparin was evaluated in a single-dose study in 27 healthy subjects receiving palifermin (60 mcg/kg) co-administered with and without therapeutic levels of unfractionated heparin. This co-administration resulted in a 5-fold increase in palifermin AUC and an 80% decrease in the mean CL. There was no significant effect of palifermin on heparin activity with respect to activated partial thromboplastin time (aPTT). The clinical relevance of this observed increase in palifermin systemic exposure is unclear [see Drug Interactions (7)].
Pharmacokinetics in Specific Populations
Renal Impairment
Results from a pharmacokinetics study in 24 subjects with varying degrees of renal impairment demonstrated that renal impairment has little or no influence on Kepivance pharmacokinetics [see Use in Specific Populations (8.6)].
Elderly
In a single-dose study, subjects received a 180-mcg/kg or 90-mcg/kg dose of palifermin administered by intravenous bolus injection. Subjects over the age of 65 (n=8) had an approximately 30% lower rate of CL on average than those 65 and younger (n=19). No dose adjustment is recommended for the geriatric population [see Use in Specific Populations (8.5)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity: No treatment-related increase in the incidence of neoplastic lesions occurred in transgenic rasH2 mice treated with 9 weekly intravenous doses of palifermin, at 167-fold higher than the recommended human dose (on a mcg/kg basis).
Mutagenicity: No clastogenic or mutagenic effects of palifermin were observed in mammalian chromosomal aberration or Ames genotoxicity assays.
Impairment of Fertility: Reproductive performance, fertility, and sperm assessment parameters were not affected when palifermin was administered intravenously to male and female rats prior to and during mating at doses up to 100 mcg/kg/day. Decreased epididymal sperm counts, and increased post-implantation losses were observed at doses ≥ 300 mcg/kg/day (5-fold higher than the recommended human dose, on a mcg/kg basis). Increased pre-implantation loss and a decreased fertility index were observed at a palifermin dose of 1000 mcg/kg/day.
13.3 Reproductive and Developmental Toxicology
In animal reproductive toxicity studies, palifermin is embryotoxic at doses that are 2.5 times (rabbits) and 5 to > 8 times (rats) the MRHD, based on body weight (mcg/kg). Pregnant rabbits received intravenous palifermin during organogenesis at doses equivalent to 1.0 and 2.5 times the MRHD, based on body weight (mcg/kg). Increased post-implantation loss and decreased fetal body weights occurred along with maternal toxicity (clinical signs and reductions in body weight gain/food consumption) at doses 2.5 times the MRHD.
In pregnant rats, animals received intravenous palifermin during organogenesis at doses of 5 to >8 times the MRHD based on body weight (mcg/kg). Increased post-implantation loss, decreased fetal body weight, and/or increased skeletal variations occurred in the presence of maternal toxicity at doses > 8 times the MRHD.
14 CLINICAL STUDIES
14.1 Autologous transplantation preparative regimens that include total body irradiation
The safety and efficacy of Kepivance in decreasing the incidence and duration of severe oral mucositis in patients with hematologic malignancies (NHL, Hodgkin's disease, acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, chronic lymphocytic leukemia, or multiple myeloma) receiving myelotoxic therapy requiring hematopoietic stem cell support, were established in a randomized placebo-controlled clinical trial of 212 patients (Study 1) and a randomized, schedule-ranging, placebo-controlled clinical trial of 169 patients (Study 2).
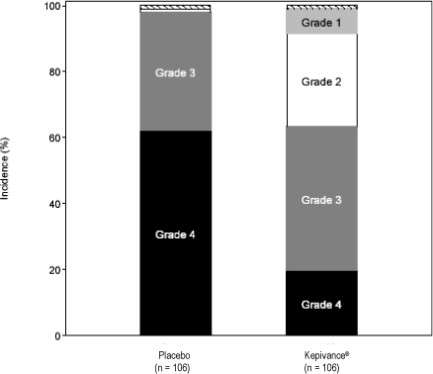
In Study 1, patients received high-dose cytotoxic therapy consisting of fractionated total-body irradiation (TBI) (12 Gy total dose), high-dose etoposide (60 mg/kg), and high-dose cyclophosphamide (100 mg/kg) followed by hematopoietic stem cell support. Patients were randomized to receive either Kepivance (n = 106) or placebo (n = 106). Kepivance 60 mcg/kg was administered as a daily intravenous injection for 3 consecutive days prior to initiation of cytotoxic therapy and for 3 consecutive days following infusion of hematopoietic stem cells. The major efficacy outcome was the number of days during which patients experienced severe oral mucositis (Grade 3/4 on the WHO [World Health Organization] scale)1. Other analyses included the incidence, duration, and severity of oral mucositis and the use of opioid analgesia. There was no evidence of a delay in time to hematopoietic recovery in patients who received Kepivance as compared to patients who received placebo. The results of Study 1 are presented in Table 2 and Figure 1.
____________________
1 WHO Oral Mucositis Scale: Grade 1 = soreness/erythema; Grade 2 = erythema, ulcers, can eat solids; Grade 3 = ulcers, requires liquid diet only; Grade 4 = alimentation not possible.
|
* P < 0.001 compared to placebo, using Generalized Cochran-Mantel-Haenszel (CMH) test stratified for study center. |
||
| Efficacy Variable |
Kepivance (60 mcg/kg/day) (n = 106) |
Placebo (n = 106) |
| Median (25th, 75th percentile) Days of WHO Grade 3/4 Oral Mucositis* | 3 (0, 6) | 9 (6, 13) |
| Incidence of WHO Grade 3/4 Oral Mucositis | 63% (67/106) | 98% (104/106) |
| Median (25th, 75th percentile) Days of WHO Grade 3/4 Oral Mucositis in Affected Patients | 6 (3, 8) (n = 67) |
9 (6, 13) (n = 104) |
| Incidence of WHO Grade 4 Oral Mucositis | 20% | 62% |
| Median (25th, 75th percentile) Cumulative Opiod Dose (morphine mg equivalents) | 212 (3, 558) | 535 (269, 1429) |
Figure 1: Study 1 Incidence of Oral Mucositis by Maximum Grade WHO Oral Mucositis Scale
Study 2 was a randomized, multi-center, placebo-controlled trial comparing varying schedules of Kepivance. All patients received high-dose cytotoxic therapy consisting of fractionated TBI (12cGy total dose), high-dose etoposide (60 mg/kg), and high-dose cyclophosphamide (75—100 mg/kg) followed by hematopoietic stem cell support. The results for Study 1 were supported by results observed in the subset of patients in Study 2 who received the same dose and schedule of Kepivance administered in Study 1. One arm of Study 2 that included patients who received Kepivance for 3 consecutive days prior to initiation of cytotoxic therapy, a dose given on the last day of TBI prior to etoposide, and for 3 consecutive days following infusion of hematopoietic stem cells was prematurely closed by the Safety Committee for lack of efficacy and a trend towards increased severity and duration of oral mucositis as compared to placebo-treated patients. The Safety Committee attributed the safety finding to Kepivance having been administered within 24 hours of chemotherapy, which resulted in an increased sensitivity of the rapidly dividing epithelial cells in the immediate post-chemotherapy period [see Dosage and Administration (2.1) and Drug Interactions (7)].
14.2 Lack of Efficacy: Autologous transplantation preparative regimen using high dose melphalan
In a post approval study, Study 3, designed to determine the efficacy of Kepivance with a high dose melphalan preparative regimen, patients with multiple myeloma were evaluated in a multicenter, randomized, double-blind, placebo-controlled trial. The conditioning regimen was melphalan (200 mg/m2) on day -2 followed by autologous hematopoietic stem cell support. A total of 281 patients were randomized to 3 arms: Kepivance before melphalan on days -6, -5, -4 and after melphalan on days 0, 1, and 2 (pre-post) (n=115); Kepivance before melphalan on days -6, -5, -4 (pre) (n=109); or placebo (n=57).
The main outcome of the study was maximum severity of WHO oral mucositis. The median age of enrolled patients was 57 years (range 32-69), and 55% were male. The results are presented in Figure 2. The prespecified primary analysis was a comparison between the Kepivance pre-post and pre arms to placebo. The incidence of WHO Grade 3 and 4 in the Kepivance pre-post arm was 38%, compared to 37% in the placebo arm. There were no significant differences between either of the Kepivance regimens and the placebo arm in the incidence of severe oral mucositis.
Figure 2: Incidence of Oral Mucositis by Maximum Grade WHO Oral Mucositis Scale in High Dose Melphalan Study
A subset of subjects enrolled in the multiple myeloma study were included in an evaluation for the risk of cataract development in patients receiving Kepivance treatment. Ophthalmologic examinations were performed on 101 patients enrolled in a double-blind, randomized, placebo-controlled study of two different schedules of Kepivance (pre and post chemotherapy and pre chemotherapy only) for reduction in severity of oral mucositis in subjects with multiple myeloma receiving high dose melphalan followed by autologous peripheral blood stem cell transplantation. For the primary cataract endpoint of incidence of cataract development or cataract progression at Month 12, there was a greater proportion of subjects that experienced cataract development in the Kepivance group: 48% (25/52) compared with the placebo group: 29% (4/14) (difference of 17 [95% CI: -11, 46]) [see Adverse Reactions - Clinical Trial Experience (6.1)].
16 HOW SUPPLIED/STORAGE AND HANDLING
Kepivance is supplied as a lyophilized powder in single use vials containing 6.25 mg of palifermin.
Kepivance vials are supplied in:
- a dispensing pack containing 3 vials (NDC 66658-112-03)
- a dispensing pack containing 6 vials (NDC 66658-112-06)
- a distribution case containing 4 dispensing packs (NDC 66658-112-24) [4 x 6 vial dispensing packs (24 x 6.25 mg/vial)].
Store Kepivance vials in the dispensing pack in its carton refrigerated at 2° to 8°C (36° to 46°F) until time of use. Protect from light.
17 PATIENT COUNSELING INFORMATION
Advise patients to report the following to healthcare providers:
- Rashes and reddening of skin [see Adverse Reactions (6.1)]
- Itchiness [see Adverse Reactions (6.1)]
- Swelling of tongue [see Adverse Reactions (6.1)]
- Changes in mouth and tongue sensation [see Adverse Reactions (6.1)]
- Alteration in taste [see Adverse Reactions (6.1)]
Inform patients
- That the safety and efficacy of Kepivance have not been established in patients with non-hematologic malignancies [see Indications and Usage (1) and Warnings and Precautions (5.1)]
- Of the evidence of tumor growth and stimulation in cell culture and in animal models of non-hematopoietic human tumors [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.1)]
sobi
SWEDISH ORPHAN BIOVITRUM
Manufactured by:
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm, Sweden
U.S. License No. 1859
© Swedish Orphan Biovitrum AB (publ). All rights reserved.
Principal Display Panel -- Vial Dispensing Packs – Label
6 x 6.25 mg/vial Single Use Vials
sobi
SWEDISH ORPHAN BIOVITRUM
Kepivance
®
(palifermin)
For Injection
6.25 mg/vial
6.25 mg/vial
For Intravenous Injection Only
Store at 2° to 8°C.
Store reconstituted solution for up to 24 hours.
Do not freeze or shake after reconstitution.
Protect from light.
Manufactured by:
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm, Sweden
License No. 1859
Kepivancepalifermin INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||