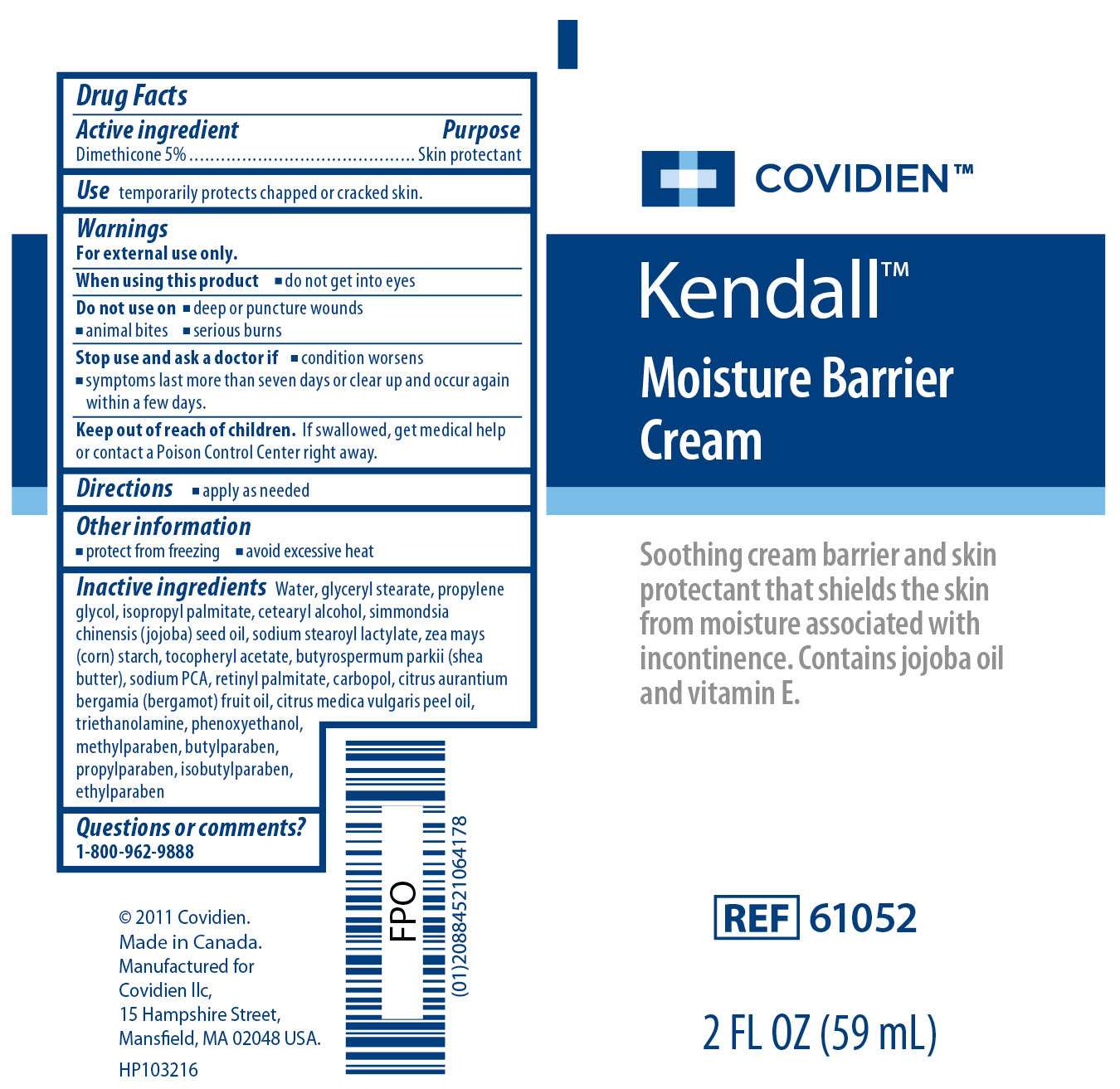
Kendall Moisture Barrier
Kendall Moisture Barrier Cream
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient
Dimethicone 5%
Purpose
Purpose
Skin Protectant
Uses
Use
temporarily protects chapped or cracked skin.
Warnings
For external use only.When using this product
- do not get into eyes
- deep or puncture wounds
- animal bites
- serious burns
- condition worsens
- symptoms last more than seven days or clear up and occur again within a few days
Directions
- apply as needed
Other Information
- protect from freezing
- avoid excessive heat
Inactive ingredients
Water, simmondsia chinensis (jojoba) seed oil, zea mays (corn) starch, cetearyl alcohol, glyceryl stearate, cirtus aurantium bergamia (bergamot) fruit oil, citrus medica vulgaris peel oil, sodium stearoyl lactylate, retinyl palmitate, tocopheryl acetate, isopropyl palmitate, propylene glycol, triethanolamine, sodium PCA, carbopol, butyrospermum parkii (shea butter), phenoxyethanol, methylparaben, butylparaben, propylparaben, isobutylparaben, ethylparaben
Questions or comments? 1-800-962-9888
Image of Kendall Moisture Barrier Cream 2 Fl oz Label
Kendall Moisture BarrierDimethicone CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||