Jurlique Purely Sun-Defying
Jurlique International Pty. Ltd.
Jurlique Purely Sun-Defying
FULL PRESCRIBING INFORMATION: CONTENTS*
- Use
- Warnings
- Directions
- Jurlique Purely Sun-Defying Other information
- Inactive ingredients
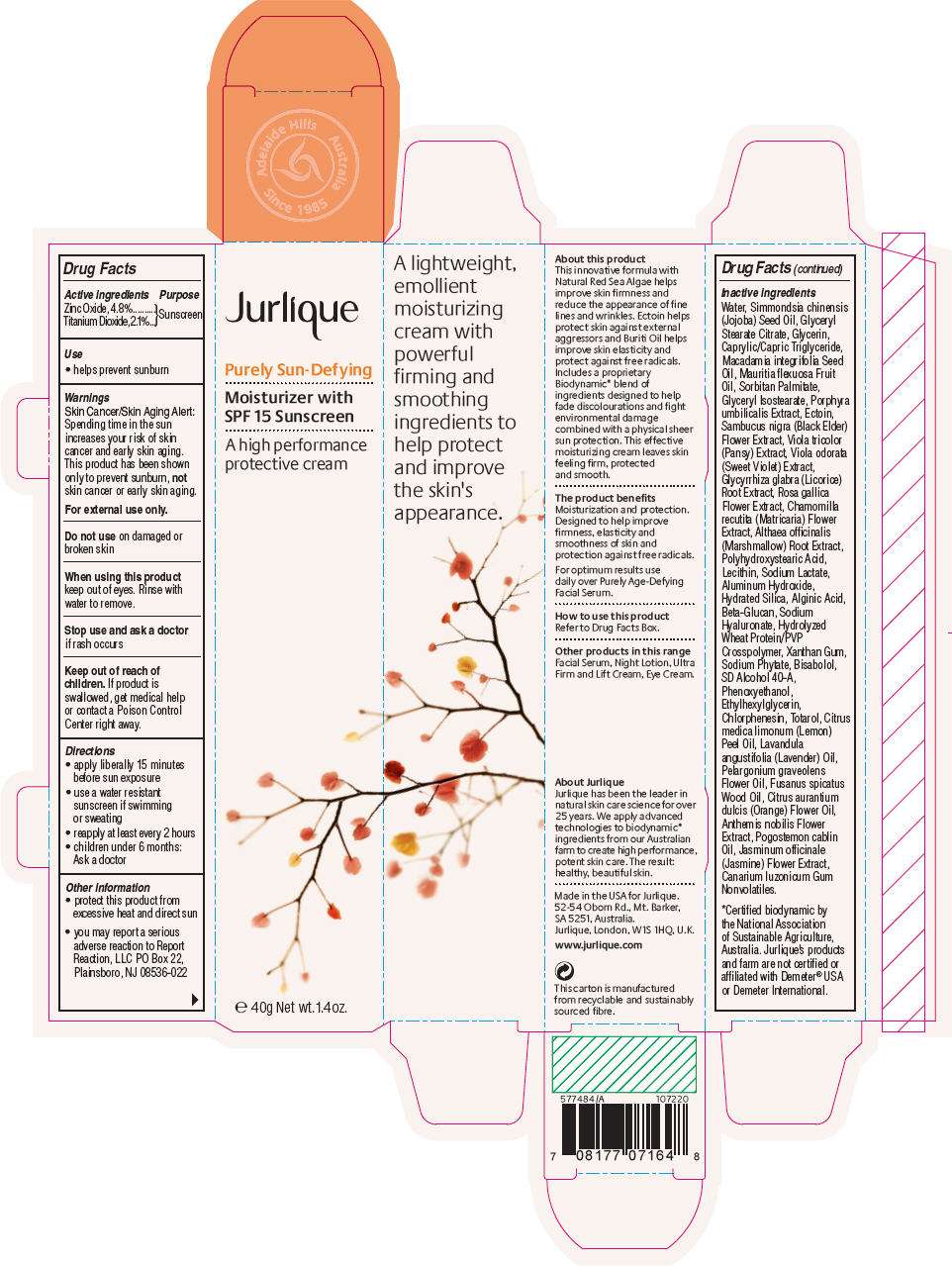
- PRINCIPAL DISPLAY PANEL - 40g Tube Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active ingredients | Purpose | |
|---|---|---|
| Zinc Oxide, 4.8% Titanium Dioxide, 2.1% |
|
Sunscreen |
Use
- helps prevent sunburn
Warnings
Skin Cancer/Skin Aging Alert
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
For external use only.
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: Ask a doctor
Jurlique Purely Sun-Defying Other information
- protect this product from excessive heat and direct sun
- you may report a serious adverse reaction to Report Reaction, LLC PO Box 22, Plainsboro, NJ 08536-022
Inactive ingredients
Water, Simmondsia chinensis (Jojoba) Seed Oil, Glyceryl Stearate Citrate, Glycerin, Caprylic/Capric Triglyceride, Macadamia integrifolia Seed Oil, Mauritia flexuosa Fruit Oil, Sorbitan Palmitate, Glyceryl Isostearate, Porphyra umbilicalis Extract, Ectoin, Sambucus nigra (Black Elder) Flower Extract, Viola tricolor (Pansy) Extract, Viola odorata (Sweet Violet) Extract, Glycyrrhiza glabra (Licorice) Root Extract, Rosa gallica Flower Extract, Chamomilla recutita (Matricaria) Flower Extract, Althaea officinalis (Marshmallow) Root Extract, Polyhydroxystearic Acid, Lecithin, Sodium Lactate, Aluminum Hydroxide, Hydrated Silica, Alginic Acid, Beta-Glucan, Sodium Hyaluronate, Hydrolyzed Wheat Protein/PVP Crosspolymer, Xanthan Gum, Sodium Phytate, Bisabolol, SD Alcohol 40-A, Phenoxyethanol, Ethylhexylglycerin, Chlorphenesin, Totarol, Citrus medica limonum (Lemon) Peel Oil, Lavandula angustifolia (Lavender) Oil, Pelargonium graveolens Flower Oil, Fusanus spicatus Wood Oil, Citrus aurantium dulcis (Orange) Flower Oil, Anthemis nobilis Flower Extract, Pogostemon cablin Oil, Jasminum officinale (Jasmine) Flower Extract, Canarium luzonicum Gum Nonvolatiles.
PRINCIPAL DISPLAY PANEL - 40g Tube Carton
Jurlique
Purely Sun-Defying
Moisturizer with
SPF 15 Sunscreen
A high performance
protective cream
e 40g Net wt. 1.4 oz.