Inflammation OTC
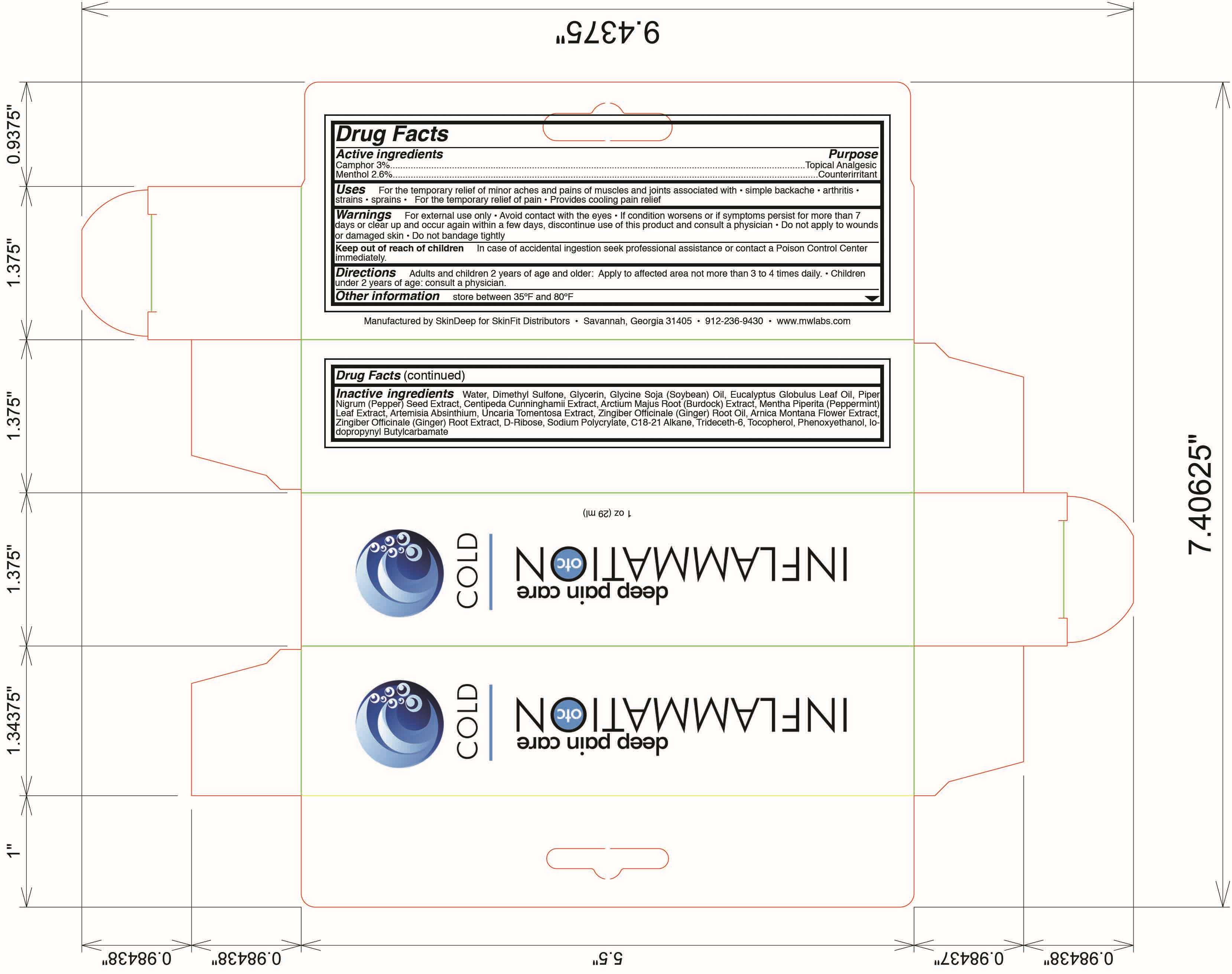
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Inflammation OTC Uses
- Warnings
- Keep out of reach of children
- Directions
- Inactive Ingredients
- Questions or Comments
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active Ingredient
Camphor (3.0%)
Menthol (2.6%)
Purpose
Topical Analgesic
Counterirritant
Inflammation OTC Uses
For temporary relief of minor aches and pains of muscles and joints associated with
- simple backache
- arthritis
- strains
- sprains
Warnings
For external use only
Avoid contact with the eyes
If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a physician
Do not apply to wounds or damaged skin
Do not bandage tightly
Keep out of reach of children
In case of accidental ingestion seek professional assistance or contact a poison control immediately
Directions
Adults and children 2 years of age and older- Apply to affected area not more than 3 to 4 times daily.
Children under 2 years of age- Consult a physician.
Inactive Ingredients
water, dimethyl sulfone, glycerin, glycine soja (soybean) oil, eucalyptus globulus oil, piper nigrum (pepper) seed extract, centipedia cunninghamii extract, arctium majus root (burdock) extract, mentha piperita (peppermint) leaf extract, artemisia absinthium, uncaria tomentosa extract, zingiber officinale (ginger) root oil, arnica montana flower extract, zingiber officinale (ginger) root extract, d-ribose, sodium polycrylate, c18-21 alkane, trideceth-6, tocopherol, phenoxyethanol, iodopropynyl butylcarbamate
Questions or Comments
Store between 35 F and 80 F
call 1-912-236-9430 weekdays
Principal Display Panel
Inflammation OTC
Deep Pain Care Cold
New wt 1.1 oz. (31 mL)
Manufactured by SkinDeep for SkinFit Distributors
Savannah, GA 31405
Inflammation OTCCamphor, Menthol LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||