Indomethacin
Camber Pharmaceuticals, Inc.
Hetero Labs Limited
Indomethacin Extended Release Capsules, USPRx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- INDOMETHACIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- INDOMETHACIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INDOMETHACIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
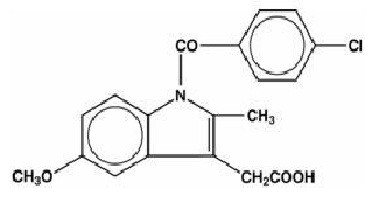
INDOMETHACIN DESCRIPTION
INDICATIONS AND USAGE .
19164
CLINICAL PHARMACOLOGY
Indomethacin is a nonsteroidal drug with anti-inflammatory, antipyretic and analgesic properties. Its mode of action, like that of other anti-inflammatory drugs, is not known. However, its therapeutic action is not due to pituitary-adrenal stimulation.
Indomethacin is a potent inhibitor of prostaglandin synthesis in vitro. Concentrations are reached during therapy which have been demonstrated to have an effect in vivo as well. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Moreover, prostaglandins are known to be among the mediators of inflammation. Since indomethacin is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
Indomethacin has been shown to be an effective anti-inflammatory agent, appropriate for long-term use in rheumatoid arthritis, ankylosing spondylitis, and osteoarthritis.
Indomethacin affords relief of symptoms; it does not alter the progressive course of the underlying disease.
Indomethacin suppresses inflammation in rheumatoid arthritis as demonstrated by relief of pain and reduction of fever, swelling and tenderness. Improvement in patients treated with indomethacin for rheumatoid arthritis has been demonstrated by a reduction in joint swelling, average number of joints involved and morning stiffness; by increased mobility as demonstrated by a decrease in walking time; and by improved functional capability as demonstrated by an increase in grip strength.
Indomethacin has been reported to diminish basal and CO2 stimulated cerebral blood flow in healthy volunteers following acute oral and intravenous administration. In one study, after one week of treatment with orally administered indomethacin, this effect on basal cerebral blood flow had disappeared. The clinical significance of this effect has not been established.
INDICATIONS & USAGE
Carefully consider the potential benefits and risks of indomethacin extended-release capsules and other treatment options before deciding to use indomethacin extended-release capsules. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ).
Indomethacin extended-release capsules have been found effective in active stages of the following:
PRECAUTIONS,Drug Interactions
INDOMETHACIN CONTRAINDICATIONS
WARNINGS: Anaphylactoid Reactions, Precautions: Preexisting Asthma
WARNINGS
WARNINGS
Cardiovascular Effects
Cardiovascular Thrombotic Events
WARNINGS , Gastrointestinal Effects
CONTRAINDICATIONS
Hypertension
Congestive Heart Failure and Edema
Gastrointestinal Effects
Risk of Ulceration,Bleeding and Perforation
peptic ulcer disease and/or gastrointestinal bleeding
Renal Effects
Advanced Renal Disease
Anaphylactoid Reactions
CONTRAINDICATIONS PRECAUTIONS::(PreexistingAsthma ).
Skin Reactions
NSAIDs, including indomethacin extended-release capsules, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
Pregnancy
PRECAUTIONS
General Precautions
Indomethacin extended-release capsules cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
The pharmacological activity of indomethacin extended-release capsules in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
Hepatic Effects
Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs, including indomethacin extended-release capsules. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test values has occured, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with indomethacin extended-release capsules. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), indomethacin extended-release capsules should be discontinued.
Hematological Effects
Preexisting Asthma
Information for Patients
Patients should be informed of the following information before initiating therapy with a NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
WARNINGS : Cardiovascular Effects
WARNINGS, Gastrointestinal EffectsRisk of Ulceration, Bleeding, and Perforation
WARNINGS
7. In late pregnancy, as with other NSAIDs, indomethacin extended-release capsules should be avoided because it will cause premature closure of the ductus arteriosus.
Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, indomethacin extended-release capsules should be discontinued.
Drug Interactions
Interactions
ACE inhibitors
Aspirin
Furosemide
WARNINGS, Renal Effects
Lithium
Methotrexate
Warfarin
Drug & OR Laboratory Test Interactions
Carcinogenesis & Mutagenesis & Impairment Of Fertility
Pregnancy
Pregnancy
Nonteratogenic Effects
Labor & Delivery
Nursing Mothers
Pediatric Use
Geriatric Use
INDOMETHACIN ADVERSE REACTIONS
|
Incidence greater than 1%
|
GASTROINTESTINAL
*
*
CENTRAL NERVOUS SYSTEM
*
SPECIALSENSES
CARDIOVASCULAR
METABOLIC
INTEGUMENTARY
HEMATOLOGIC
HYPERSENSITIVITY
GENITOURINARY
MISCELLANEOUS
|
Incidence less than 1%
|
GASTROINTESTINAL
CENTRAL NERVOUS SYSTEM
SPECIAL SENSES
CARDIOVASCULAR
METABOLIC
INTEGUMENTARY
HEMATOLOGIC
HYPERSENSITIVITY
GENITOURINARY
MISCELLANEOUS
*Reactions occurring in 3% to 9% of patients treated with indomethacin. (Those reactions occurring in less than 3% of the patients are unmarked.)
Causal Relationship Unknown
PRECAUTIONS, General
Cardiovascular
Hematologic : Although there have been several reports of leukemia, the supporting information is weak.
Genitourinary
: Urinary frequency
OVERDOSAGE
50
DOSAGE & ADMINISTRATION
WARNINGS CLINICAL PHARMACOLOGY
Pediatric Use WARNINGS
Adult Use
Suggested Dosage
The following information refers to Extended-release Indomethacin Capsules (75 mg):
HOW SUPPLIED
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from moisture
SPL MEDGUIDE
Indomethacin Extended-release Capsules, USP
MEDICATION GUIDE
for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
See the end of this Medication Guide for a list of prescription NSAID medicines
What is the most important information I should know about medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines may increase the chance of a heart attack or stroke that can lead to death
NSAID medicines should never be used right before or after a heart surgery called a "coronary artery bypass graft (CABG)."
NSAID medicines can cause ulcers and bleeding in the stomach and intestines at any time during treatment. Ulcers and bleeding:
The chance of a person getting an ulcer or bleeding increases with:
NSAID medicines should only be used:
What are Non-Steroidal Anti-Inflammatory Drugs (NSAIDS)?
Who should not take a Non-Steroidal Anti-Inflammatory Drug (NSAID)?
Do not take an NSAID medicine:
Tell your healthcare provider:
Keep a list of your medicines to show to your healthcare provider and pharmacist
NSAID medicines should not be used by pregnant women late in their pregnancy
Talk to your doctor
What are the possible side effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDS)?
|
Serious side effects include:
|
Other side effects include:
|
| · heart attack · stroke · high blood pressure · heart failure from body swelling (fluid retension) · kidney problems including kidney failure · bleeding and ulcers in the stomach · and intestine · low red blood cells (anemia) · life-threatening skin reactions · life-threatening allergic reactions · liver problems including liver failure · asthma attacks in people who have asthma |
· stomach pain · constipation · diarrhea · gas · heartburn · nausea · vomiting · dizziness |
Get emergency help right away if you have any of the following symptoms:
Stop your NSAID medicine and call your healthcare provider right away if you have any of the following symptoms:
These are not all the side effects with NSAID medicines. Talk to your healthcare provider or pharmacist for more information about NSAID medicines.
Other information about Non-Steroidal Anti-Inflammatory Drugs (NSAIDS)
· Aspirin is an NSAID medicine but it does not increase the chance of a heart attack.Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can alsocause ulcers in the stomach and intestines.
· Some of these NSAID medicines are sold in lower doses without a prescription(over-the-counter). Talk to your healthcare provider before using over-the-counterNSAIDS for more than 10 days.
NSAID medicines that need a prescription
|
Generic Name |
Trade name |
| Celecoxib |
Celebrex |
| Diclofenac |
Cataflam, Voltaren, Arthrotec (combined with misoprostol) |
| Diflunisal |
Dolobid |
| Etodolac |
Lodine, Lodine XL |
| Fenoprofen |
Nalfon, Nalfon 200 |
| Flurbiprofen |
Ansaid |
| Ibuprofen |
Motrin, Tab-Profen, Vicoprofen (combined with hydrocodone), Combunox (combined with oxycodone) |
| Indomethacin |
Indocin, Indocin SR, Indo-Lemmon, Indomethegan |
| Ketoprofen |
Oruvail |
| Ketorolac |
Toradol |
| Mefenamic Acid |
Ponstel |
| Meloxicam |
Mobic |
| Nabumetone |
Relafen |
| Naproxen |
Naprosyn, Anaprox, Anaprox DS, EC-Naprosyn, Naprelan, Naprapac (copackaged with lansoprazole) |
| Oxaprozin |
Daypro |
| Piroxicam |
Feldene |
| Sulindac |
Clinoril |
| Tolmetin |
Tolectin, Tolectin DS, Tolectin 600 |
This Medication Guide has been approved by the U.S Food and Drug Administration.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Manufactured for:
Camber Pharmaceuticals, Inc. 2017702
Piscataway, NJ 08854
By: HETEROTM
Hetero Labs Limited
Jeedimetla, Hyderabad – 500 055, India.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
IndomethacinIndomethacin CAPSULE, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||