Indomethacin
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- INDOMETHACIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- INDOMETHACIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INDOMETHACIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
INDOMETHACIN DESCRIPTION
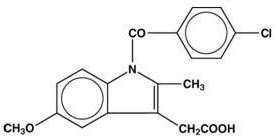
C19H16ClNO4 M.W. 357.79
Indomethacin, USP is practically insoluble in water and sparingly soluble in alcohol. It has a pKa of 4.5 and is stable in neutral or slightly acidic media and decomposes in strong alkali.
Each capsule for oral administration contains 25 mg or 50 mg of indomethacin and the following inactive ingredients: lactose monohydrate, sodium lauryl sulphate, sodium starch glycolate, colloidal silicon dioxide, magnesium stearate. The hard gelatin shell consists of gelatin, titanium dioxide USP, FD & C Blue 1, D & C Yellow 10. The capsules are printed with black ink containing black iron oxide E172 dye.
CLINICAL PHARMACOLOGY
INDICATIONS AND USAGE
INDICATIONS & USAGE
WARNINGS
INDOMETHACIN CONTRAINDICATIONS
DESCRIPTION
WARNINGS: Anaphylactic / Anaphylactoid ReactionsPRECAUTIONS: General: Preexisting Asthma
WARNINGS
WARNINGS
Cardiovascular Effects
WARNINGS: Gastrointestinal Effects
CONTRAINDICATIONS
Gastrointestinal Effects
Renal Effects
PRECAUTIONS: Drug Interactions
Anaphylactic / Anaphylactoid Reactions
CONTRAINDICATIONSPRECAUTIONS: General: Preexisting Asthma
Skin Reactions
Pregnancy
Ocular Effects
Central Nervous System Effects
PRECAUTIONS
General Precautions
Information for Patients
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
WARNINGS: Cardiovascular Effects
WARNINGS: Gastrointestinal Effects: Risk of Ulceration, Bleeding, and Perforation
WARNINGS
Laboratory Tests
Drug Interactions
WARNINGS: Renal Effects
Drug & OR Laboratory Test Interactions
Carcinogenesis & Mutagenesis & Impairment Of Fertility
Pregnancy
Labor & Delivery
Nursing Mothers
Pediatric Use
Geriatric Use
WARNINGS , Gastrointestinal Effects: Risk of Ulceration, Bleeding, and PerforationDOSAGE AND ADMINISTRATIONWARNINGS: Gastrointestinal Effects: Risk of Ulceration, Bleeding, and Perforation
ADVERSE REACTIONS
WARNINGS: Renal Effects
INDOMETHACIN ADVERSE REACTIONS
|
Incidence greater than 1%
|
GASTROINTESTINAL
CENTRAL NERVOUS SYSTEM
SPECIAL SENSES
CARDIOVASCULAR
METABOLIC
INTEGUMENTARY
HEMATOLOGIC
HYPERSENSITIVITY
GENITOURINARY
MISCELLANEOUS
|
Incidence less than 1%
|
GASTROINTESTINAL
CENTRAL NERVOUS SYSTEM
SPECIAL SENSES
CARDIOVASCULAR
METABOLIC
INTEGUMENTARY
HEMATOLOGIC
HYPERSENSITIVITY
GENITOURINARY
MISCELLANEOUS
Causal Relationship Unknown
Cardiovascular:
Hematologic:
Genitourinary:
PRECAUTIONS: General
OVERDOSAGE
DOSAGE & ADMINISTRATION
WARNINGS
Pediatric Use
WARNINGS
Adult Use
PRECAUTIONS: Geriatric Use
HOW SUPPLIED
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Protect from light.
PHARMACIST:

SPL MEDGUIDE
INDOMETHACIN CAPSULES USP
Medication Guide for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
See the end of this Medication Guide for a list of prescription NSAID medicines
What is the most important information I should know about medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines may increase the chance of a heart attack or stroke that can lead to death.
NSAID medicines should never be used right before or after a heart surgery called a "coronary artery bypass graft (CABG)."
NSAID medicines can cause ulcers and bleeding in the stomach and intestines at any time during treatment. Ulcers and bleeding:
The chance of a person getting an ulcer or bleeding increases with:
NSAID medicines should only be used:
What are Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
Who should not take a Non-Steroidal Anti-Inflammatory Drug (NSAID)?
Do not take an NSAID medicine:
Tell your healthcare provider:
Keep a
list of your medicines to show to your healthcare provider and pharmacist.
NSAID medicines should not be used by pregnant women late in their pregnancy
Talk to your doctor.
What are the possible side effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
| serious side effects include: | Other side effects include: |
|
• heart attack • stroke • high blood pressure • heart failure from body swelling (fluid Retension) • kidney problems including kidney failure • bleeding and ulcers in the stomach and intestine • low red blood cells (anemia) • life-threatening skin reactions • life-threatening allergic reactions • liver problems including liver failure • asthma attacks in people who have asthma |
• stomach pain • constipation • diarrhea • gas • heartburn • nausea • vomiting • dizziness |
Get emergency help right away if you have any of the following symptoms:
Stop your NSAID medicine and call your healthcare provider right away if you have any of the following symptoms:
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
NSAID medicines that need a prescription
| Generic Name | Trade name |
|
Celecoxib Diclofenac Diflunisal Etodolac Fenoprofen Flurbiprofen Ibuprofen Indomethacin Ketoprofen Ketorolac MefenamicAcid Meloxicam Nabumetone Naproxen Oxaprozin Piroxicam Sulindac Tolmetin |
Celebrex Cataflam, Voltaren, Arthrotec (combined with misoprostol) Dolobid Lodine, Lodine XL Nalfon, Nalfon 200 Ansaid Motrin, Tab-Profen, Vicoprofen*(combined with hydrocodone), Combunox (combined with oxycodone) Indocin, Indocin SR, Indo-Lemmon, Indomethagan Oruvail Toradol Ponstel Mobic Relafen Naprosyn, Anaprox, Anaprox DS, EC-Naprosyn, Naprelan, Naprapac (copackaged with lansoprazole) Daypro Feldene Clinoril Tolectin, Tolectin DS, Tolectin 600 |
*
This Medication Guide has been approved by the U.S. Food and Drug Administration.

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL


IndomethacinIndomethacin CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
IndomethacinIndomethacin CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!