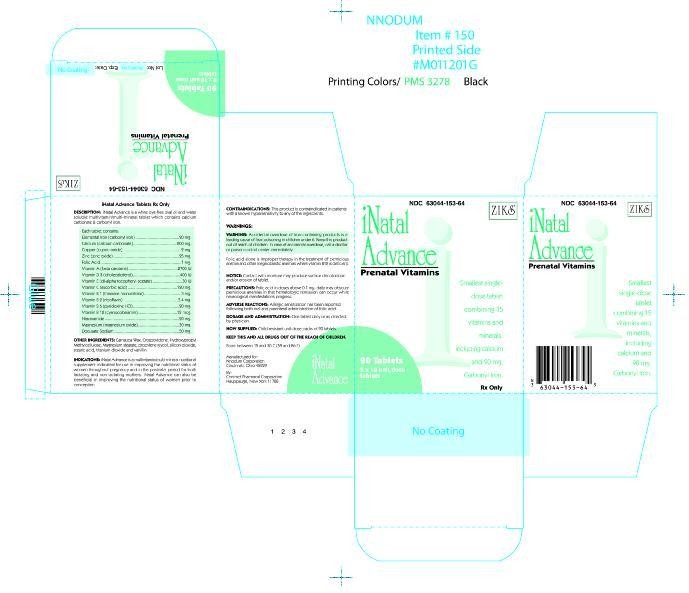
INATAL ADVANCE
INATAL ADVANCE Tablet 63044-153-64
FULL PRESCRIBING INFORMATION
Prenatal multivitamin/multimineral tablet
combining 15 vitamins and minerals,
including calcium and carbonyl iron.
Rx Only
| WARNING: Accidental overdose of iron containing prod-ucts is a leading cause of fatal poisoning in children under 6. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or poison controll center immediately. |
INATAL Advanced ® is a white, dye-free, oval shaped, oil-and-water soluble multivitamin/multimineral tablet which contains calcium carbonate and carbonyl iron.
Each tablet contains:
Vitamin A (as beta-carotene) . . . . . . . . . . . 2700 I.U.
Vitamin C (ascorbic acid) . . . . . . . . . . . . . . 120 mg
Calcium (as calcium carbonate) . . . . . . . . . . 200 mg
Elemental Iron (as carbonyl iron) . . . . . . . . . . 90 mg
Vitamin D3 (cholecalciferol) . . . . . . . . . . . . 400 I.U.
Vitamin E (dl-alpha tocopheryl acetate) . . . . . 30 I.U.
Vitamin B1 (as thiamine mononitrate) . . . . . . . . 3 mg
Vitamin B2 (riboflavin, USP) . . . . . . . . . . . . . 3.4 mg
Niacinamide . . . . . . . . . . . . . . . . . . . . . . . . 20 mg
Vitamin B6 (as pyridoxine HCI, USP) . . . . . . . 20 mg
Folic Acid, USP . . . . . . . . . . . . . . . . . . . . . . . 1 mg
Vitamin B12 (cyanocobalamin) . . . . . . . . . . . 12 mcg
Zinc (as zinc oxide, USP) . . . . . . . . . . . . . . . . 25 mg
Copper (as cupric oxide) . . . . . . . . . . . . . . . . . 2 mg
Magnesium (as magnesium oxide, USP) . . . . . 30 mg
Docusate Sodium . . . . . . . . . . . . . . . . . . . . 50 mg
INACTIVE INGREDIENTS: Carnauba wax, crospovidone, ethyl vanillin, hydroxypropyl methylcellulose, magnesium stearate, polyethylene glycol, silicon dioxide, stearic acid, and titanium dioxide
INATAL ADVANCE ® is a multivitamin/multimineral nutritional supplement indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers. INATAL Advanced® can also be beneficial in improving the nutritional status of women prior to conception.
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient.
NOTICE: Contact with moisture may produce surface discoloration or erosion of the tablet.
Folic acid in doses above 0.1mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
Safety and effectiveness in pediatric patients have not been established
.
Clinical studies on this product have not been performed in sufficient numbers of subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually staring at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
One tablet daily or as directed by a physician.
NDC 63044-153-64 Unit Dose Packs of 90’s with each blister card containing 10 tablets per card.
Store at controlled room temperature 15°- 30°C (59°-86°F).
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Manufactured For
Nnodum Pharmaceuticals
Cincinnati, Ohio 45229

INATAL ADVANCEINATAL ADVANCE TABLET, COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||