Icy Hot
Icy Hot Medicated Sleeve
FULL PRESCRIBING INFORMATION
Drug Facts
Icy Hot Medicated Sleeve - Small
Menthol 16%
Topical analgesic
temporarily relieves minor pain associated with:
- arthritis
- bursitis
- tendonitis
- muscle strains
- sprains
- bruises
- cramps
For external use only
- use only as directed
- do not bandage tightly over sleeve or use with a heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
ask a health professional before use.
If swallowed, get medical help or contact a Poison Control Center right away.
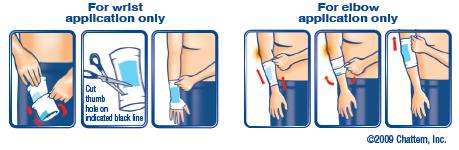
adults and children over 12 years:
- remove plastic insert and discard
- pull ICY HOT® SLEEVE onto ankle, elbow or wrist – note: medicine is away from skin
- carefully pull ICY HOT® SLEEVE over itself, medicine will then be touching the skin
note: the drawing (above) should help you understand how to do this
- apply one ICY HOT® SLEEVE to affected area
- repeat as necessary, but no more than 3 times daily
- sleeve should feel snug but not tight
children 12 years or younger: ask a doctor
cetyl alcohol, diethylene glycol monoethyl ether, diisopropyl adipate, disodium EDTA, glycerin, glyceryl dilaurate, glyceryl stearate, menthyl lactate, methylparaben, PEG-150 stearate, phenoxyethanol, polysorbate 80, soya sterol, water, xanthan gum (may contain citric acid) (245-39)
Drug Facts
Icy Hot Medicated Sleeve - Large
Menthol 16%
Topical analgesic
temporarily relieves minor pain associated with:
- arthritis
- bursitis
- tendonitis
- muscle strains
- sprains
- bruises
- cramps
For external use only
- use only as directed
- do not bandage tightly over sleeve or use with a heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
ask a health professional before use.
If swallowed, get medical help or contact a Poison Control Center right away.
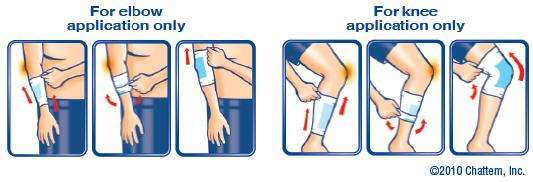
adults and children over 12 years:
- remove plastic insert and discard
- pull ICY HOT® SLEEVE onto ankle, elbow or knee – note: medicine is away from skin
- carefully pull ICY HOT® SLEEVE over itself, medicine will then be touching the skin
note: the drawing (above) should help you understand how to do this
- apply one ICY HOT® SLEEVE to affected area
- repeat as necessary, but no more than 3 to 4 times daily
- sleeve should feel snug but not tight
children 12 years or younger: ask a doctor
cetyl alcohol, citric acid, diisopropyl adipate, disodium EDTA, ethoxydiglycol, glycerin, glyceryl dilaurate, glyceryl stearate, glycine soja sterols, menthyl lactate, methylparaben, PEG-150 stearate, phenoxyethanol, polysorbate 80, water, xanthan gum (283-110)
ICY HOT® MEDICATED SLEEVE
Menthol 16%
SMALL
Ankles, Elbows and Wrists
Contains 3 Individually Wrapped Sleeves
Expands to fit 5” to 12” in circumference

ICY HOT® MEDICATED SLEEVE
Menthol 16%
LARGE
Ankles, Elbows and Knees
Contains 3 Individually Wrapped Sleeves
Expands to fit 8” to 24” in circumference

Icy HotMenthol PATCH
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Icy HotMenthol PATCH
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||