HYPAQUE
HYPAQUE-76(Diatrizoate Meglumine and Diatrizoate Sodium Injection, USP)76%
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYPAQUE DESCRIPTION
- CLINICAL PHARMACOLOGY
- HYPAQUE INDICATIONS AND USAGE
- HYPAQUE CONTRAINDICATIONS—General
- WARNINGS—General
- PRECAUTIONS—General
- HYPAQUE ADVERSE REACTIONS—General
- OVERDOSAGE
- HYPAQUE DOSAGE AND ADMINISTRATION—General
- INDIVIDUAL HYPAQUE INDICATIONS AND USAGE
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
Sterile Aqueous Injection
For Excretory Urography
Aortography
Angiocardiography (Ventriculography, Pulmonary
Angiography, Selective Coronary Arteriography)
Peripheral Angiography (Peripheral Arteriography
and Peripheral Venography)
Intravenous Digital Arteriography
Contrast Enhancement of Computed
Tomographic Head Imaging
Contrast Enhancement of Computed
Tomographic Body Imaging
Selective Renal Arteriography
Selective Visceral Arteriography
Central Venography
Renal Venography
| NOT FOR INTRATHECAL USE |
HYPAQUE DESCRIPTION
HYPAQUE-76, brand of diatrizoate meglumine and diatrizoate sodium, is a water-soluble, radiopaque diagnostic medium. It is supplied as a 76 percent sterile aqueous solution providing 66 percent (w/v) diatrizoate meglumine and 10 percent (w/v) diatrizoate sodium. It is a triiodinated benzoic acid derivative containing 37 percent (w/v) organically bound iodine. Each mL contains 370 mg iodine and 3.68 mg (0.16 mEq) sodium. It is constituted as a radiopaque iodinated anion, diatrizoate, and the radiolucent cations meglumine and sodium. It is the organically bound iodine of the anion which opacifies internal structures for x-ray visualization and fluoroscopy.
The solution is hypertonic to blood with an osmolality of 2016 mosm/kg (determined by VPO). The viscosity is 9 cp at 37° C. The pH is adjusted between 6.0 and 7.7 using Na2CO3 with HCl or NaOH. The pKa is 3.4 for diatrizoic acid. Edetate calcium disodium 0.01 percent has been added as a sequestering stabilizing agent. The solution is clear, colorless to pale yellow.
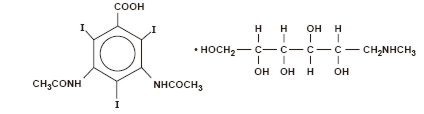
Diatrizoate meglumine is 1-deoxy-1-(methylamino)-D-glucitol 3, 5-diacetamido-2,4,6-triiodobenzoate (salt) and has the following structural formula:
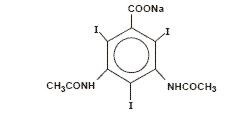
Diatrizoate sodium is monosodium, 3, 5-diacetamido-2,4,6-triiodobenzoate and has the following structural formula:
CLINICAL PHARMACOLOGY
Intravascular injection of a radiopaque diagnostic agent opacifies those vessels in the path of the flow of the contrast medium, permitting radiographic visualization of the internal structures of the human body until significant hemodilution occurs.
At physiologic pH, the water-soluble contrast media are completely dissociated into a radiopaque anion and a solubilizing cation. While circulating in tissue fluids, the compound remains ionized. However, it is not metabolized but excreted unchanged in the urine, each diatrizoate molecule remaining "obligated" to its sodium or meglumine moiety.
Following intravenous injection, the radiopaque diagnostic agents are immediately diluted in the circulating plasma. Equilibrium is reached with the extracellular compartment at about 10 minutes. Hence, the plasma concentration at 10 minutes is closely related to the dose corrected to body size.
The pharmacokinetics of the intravenously administered radiopaque contrast media are usually best described by a two compartment model with a rapid alpha phase for drug distribution and a slow beta phase for drug elimination. In patients with normal renal function, the alpha and beta half-lives were respectively 30 minutes and 120 minutes for diatrizoate. But in patients with renal functional impairment, the elimination half-life for the beta phase can be prolonged up to several days.
Injectable radiopaque diagnostic agents are excreted either through the liver or through the kidneys. These two excretory pathways are not mutually exclusive, but the main route of excretion seems to be governed by the affinity of the contrast medium for serum albumin. From 0% to 10% of diatrizoate sodium is bound to serum protein.
Diatrizoate salts are excreted unchanged predominantly through the kidneys by glomerular filtration. The amount excreted by the kidney during any period of time is determined by the filtered load; i.e., the product of plasma contrast media concentration and glomerular filtration rate. The plasma concentration is dependent upon the dose administered and the body size. The glomerular filtration rate varies with the body size, sex, age, circulatory dynamics, diuretic effect of the drug, and renal function. In patients with normal renal function the maximum urinary concentration of diatrizoate meglumine and diatrizoate sodium occurs within 10 minutes with 12 percent of the administered dose being excreted. The mean values of cumulative urinary excretion for diatrizoate meglumine and diatrizoate sodium expressed as percentage of administered dose are 38 percent at 60 minutes, 45 percent at 3 hours, and 94 to 100 percent at 24 hours.
Urinary excretion of contrast media is delayed in infants younger than 1 month and in patients with urinary tract obstruction. The urinary iodine concentration is higher with the sodium salt of diatrizoic acid than with the meglumine salt.
The liver and small intestine provide the major alternate route of excretion for diatrizoate. In patients free of severe renal disease, the fecal recovery is less than 2 percent of the administered dose. In patients with severe renal impairment the excretion of these contrast media through the gallbladder and into the small intestine sharply increases; up to 20 percent of the administered dose has been recovered in the feces in 48 hours.
Saliva is a minor secretory pathway for injectable radiopaque diagnostic agents. In patients with normal renal function, minimal amounts of contrast media are secreted unchanged. However, in uremic patients small amounts of free iodides resulting from deiodination prior to administration or in vivo, have been detected in the saliva.
Diatrizoate salts cross the placental barrier in humans by simple diffusion and appear to enter fetal tissue passively. No apparent harm to the fetus was observed when diatrizoate sodium and diatrizoate meglumine were injected intravenously 24 hours prior to delivery. However, abnormal neonatal opacification of the small intestine and colon were detected 4 to 6 days after delivery. Procedures including radiation involve a certain risk related to the exposure of the fetus. (See PRECAUTIONS—General, Pregnancy Category C.)
Injectable radiopaque diagnostic agents are excreted unchanged in human milk. (See PRECAUTIONS—General, Nursing Mothers.)
Computed Tomography
HYPAQUE-76 enhances computed tomographic brain scanning through augmentation of radiographic efficiency. The degree of enhancement of visualization of tissue density is directly related to the iodine content in an administered dose; peak iodine blood levels occur immediately following rapid injection of the dose. These levels fall rapidly within five to ten minutes. This can be accounted for by the dilution in the vascular and extracellular fluid compartments which causes an initial sharp fall in plasma concentration. Equilibration with the extracellular compartments is reached in about ten minutes; thereafter, the fall becomes exponential. Maximum contrast enhancement frequently occurs after peak blood iodine levels are reached. The delay in maximum contrast enhancement can range from five to forty minutes, depending on the peak iodine levels achieved and the cell type of the lesion. This lag suggests that radiographic contrast enhancement is at least in part dependent on the accumulation of iodine within the lesion and outside the blood pool, although the mechanism by which this occurs is not clear. The radiographic enhancement of nontumoral lesions, such as arteriovenous malformations and aneurysms, is probably dependent on the iodine content of the circulating blood pool.
In brain scanning, HYPAQUE-76, brand of diatrizoate meglumine and diatrizoate sodium injection, does not accumulate in normal brain tissue due to the presence of the blood-brain barrier. The increase in x-ray absorption in normal brain is due to the presence of contrast agent within the blood pool. A break in the blood-brain barrier such as occurs in malignant tumors of the brain allows the accumulation of the contrast medium within the interstitial tumor tissue. Adjacent normal brain tissue does not contain the contrast medium.
In nonneural tissues (during computed tomography of the body), diatrizoate diffuses rapidly from the vascular into the extravascular space. Increase in x-ray absorption is related to blood flow, concentration of the contrast medium, and extraction of the contrast medium by interstitial tumor tissue since no barrier exists. Contrast enhancement is thus due to the relative differences in extravascular diffusion between normal and abnormal tissue, quite different from that in the brain.
The pharmacokinetics of diatrizoate in both normal and abnormal tissue have been shown to be variable. Contrast enhancement appears to be greatest soon after administration of the contrast medium, and following intra-arterial rather than intravenous administration. The greatest enhancement can be detected by a series of consecutive two- to three-second scans performed just after injection (within 30 to 90 seconds), i.e., dynamic computed tomographic scanning.
Effects of Steroid Therapy
The anti-inflammatory and antiedema effects in patients receiving steroid therapy have interfered with the expected distribution of CT tissue enhancement on the scan in certain diseases.
HYPAQUE INDICATIONS AND USAGE
HYPAQUE-76 is indicated for excretory urography, aortography, angiocardiography (ventriculography, pulmonary angiography, selective coronary arteriography), peripheral angiography (peripheral arteriography and peripheral venography), intravenous digital arteriography, contrast enhancement of computed tomographic head imaging, contrast enhancement of computed tomographic body imaging, selective renal arteriography, selective visceral arteriography, central venography, and renal venography.
In addition to the following generalCONTRAINDICATIONS, WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS, there are additional listings in these categories under the particular procedures.
CONTRAINDICATIONS—General
HYPAQUE-76 has no absolute contraindications in its recommended uses (see general WARNINGS and PRECAUTIONS).
Do not use HYPAQUE-76 for myelography or for examination of dorsal cysts or sinuses which might communicate with the subarachnoid space. Even a small amount in the subarachnoid space may produce convulsions and result in fatality. (See also AORTOGRAPHY, Warnings. ) Epidural injection is also contraindicated.
HYPAQUE-76 should not be injected directly into the carotid, vertebral, or spinal arteries.
HYPAQUE-76 is contraindicated in patients with a hypersensitivity to salts of diatrizoic acid.
Urography is contraindicated in patients with anuria.
Urography and large dose vascular procedures are contraindicated in dehydrated azotemic patients. (See also PRECAUTIONS—General.)
WARNINGS—General
SEVERE ADVERSE EVENTS - INADVERTENT INTRATHECAL ADMINISTRATION
Serious adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use. These serious adverse reactions include: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Special attention must be given to insure that this drug product is not administered intrathecally.
Ionic iodinated contrast media inhibit blood coagulation, in vitro, more than nonionic contrast media. Nonetheless, it is prudent to avoid prolonged contact of blood with syringes containing ionic contrast media.
Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke have been reported during angiographic procedures with both ionic and nonionic contrast media. Therefore, meticulous intravascular administration technique is necessary, particularly during angiographic procedures, to minimize thromboembolic events. Numerous factors, including length of procedure, catheter and syringe material, underlying disease state and concomitant medications may contribute to the development of thromboembolic events. For these reasons, meticulous angiographic techniques are recommended including close attention to guidewire and catheter manipulation, use of manifold systems and/or three-way stopcocks, frequent catheter flushing with heparinized saline solutions and minimizing the length of the procedure. The use of plastic syringes in place of glass syringes has been reported to decrease but not eliminate the likelihood of in vitro clotting.
Excretory urography is potentially hazardous in patients with multiple myeloma. In some of those patients, therapeutically resistant anuria resulting in progressive uremia, renal failure, and eventually death has followed this procedure. Although neither the contrast agent nor dehydration has been proved separately to be the cause of anuria in myelomatous patients, it has been speculated that the combination of both may be causative. The risk of excretory urography in myelomatous patients is not a contraindication to the procedure; however, they require special precautions. Partial dehydration in the preparation of these patients for the examination is not recommended since this may predispose to the precipitation of myeloma protein in the renal tubules. Myeloma, which occurs most commonly in persons over age 40, should be considered before instituting urographic procedures.
Contrast media may promote sickling in individuals who are homozygous for sickle cell disease when the material is injected intravenously or intra-arterially.
Administration of radiopaque materials to patients known or suspected of having pheochromocytoma should be performed with extreme caution. If, in the opinion of the physician, the possible benefits of such procedures outweigh the considered risks, the procedures may be performed; however, the amount of radiopaque medium injected should be kept to an absolute minimum. The blood pressure should be assessed throughout the procedure and measures for treatment of a hypertensive crisis should be available.
Recent reports of thyroid storm occurring following the intravascular use of iodinated radiopaque diagnostic agents in patients with hyperthyroidism or with an autonomously functioning thyroid nodule suggest that this additional risk be evaluated in such patients before use of diatrizoate salts.
Contrast media administered for cardiac catheterization and angiocardiography may cause cellular injury to circulating lymphocytes. Chromosomal damage in humans includes inhibition of mitosis, increases in the number of micronuclei, and chromosome aberrations. The damages appear to be related to the contrast medium itself rather than to the x-ray radiation. It is to be noted that those agents have not been adequately tested in animal or laboratory systems.
Urography should be performed with caution in patients with severely impaired renal function and patients with combined renal and hepatic disease.
Subcutaneous extravasation causes transitory stinging, chiefly because of hypertonic cellulitis. If the volume extravasated is small, ill effects are very unlikely. However, if the extravasation is extensive, especially in poorly vascularized areas (e.g., dorsum of the foot or hand), and especially in the presence of vascular disease, skin slough may occur. Injection of sterile water to dilute or addition of spreading agents to speed absorption have not been successful and may aggravate the condition.
Selective spinal arteriography or arteriography of trunks providing spinal branches can cause mild to severe muscle spasm. However, serious neurologic sequelae, including permanent paralysis, could occur with even small doses of the 76 percent concentration.
In patients with subarachnoid hemorrhage, a rare association between contrast administration and clinical deterioration, including convulsions and death, has been reported. Therefore, administration of intravascular iodinated ionic contrast media in these patients should be undertaken with caution.
PRECAUTIONS—General
Diagnostic procedures which involve the use of radiopaque diagnostic agents should be carried out under the direction of personnel with the prerequisite training and with a thorough knowledge of the particular procedure to be performed. Appropriate facilities should be available for coping with any complication of the procedure, as well as for emergency treatment of severe reactions to the contrast agent itself. After parenteral administration of a radiopaque agent, competent personnel and emergency facilities should be available for at least 30 to 60 minutes since severe delayed reactions have occurred (see ADVERSE REACTIONS—General).
The possibility of a reaction, including serious, life-threatening, fatal, anaphylactic or cardiovascular reactions should always be considered (see ADVERSE REACTIONS). It is of utmost importance that a course of action be carefully planned in advance for immediate treatment of serious reactions, and that adequate and appropriate personnel be readily available in case of any reaction.
The possibility of an idiosyncratic reaction in susceptible patients should always be considered (see ADVERSE REACTIONS—General). The susceptible population includes patients with a history of a previous reaction to a contrast media, patients with a known sensitivity to iodine per se, and patients with a known clinical hypersensitivity: bronchial asthma, hay fever, and food allergies.
The occurrence of severe idiosyncratic reactions has prompted the use of several pretesting methods. However, pretesting cannot be relied upon to predict severe reactions and may itself be hazardous for the patient. It is suggested that a thorough medical history with emphasis on allergy and hypersensitivity, prior to the injection of any contrast media, may be more accurate than pretesting in predicting potential adverse reactions.
A positive history of allergies or hypersensitivity does not arbitrarily contraindicate the use of a contrast agent, where a diagnostic procedure is thought essential, but caution should be exercised (see ADVERSE REACTIONS—General). Premedication with antihistamines or corticosteroids to avoid or minimize possible allergic reactions in such patients should be considered. Recent reports indicate that such pretreatment does not prevent serious life-threatening reactions, but may reduce both their incidence and severity.
Preparatory dehydration for angiography and CT procedures is unnecessary and may be dangerous, contributing to acute renal failure in infants, young children, the elderly, patients with preexisting renal insufficiency, patients with advanced vascular disease, and diabetic patients. Dehydration in these patients seems to be enhanced by the osmotic diuretic action of urographic agents. Overnight fluid restriction for urography may be undesirable and is considered unnecessary when using this relatively high (76%) concentration.
Although azotemia is not a contraindication, the medium should be used with great care in patients with advanced renal destruction associated with severe uremia. (See also EXCRETORY UROGRAPHY, Precautions. )
Acute renal failure has been reported in diabetic patients with diabetic nephropathy and in susceptible nondiabetic patients (often elderly with preexisting renal disease) following excretory urography. Therefore, careful consideration of the potential risks should be given before performing this radiographic procedure in these patients. (See also EXCRETORY UROGRAPHY, Precautions—Preparatory Dehydration. )
Immediately following surgery, excretory urography should be used with caution in renal transplant recipients.
Due to the transitory increase in the circulatory osmotic load, injections of urographic agents should be used with caution in patients with congestive heart failure. Such patients should be observed for several hours following the procedure to detect delayed hemodynamic disturbances.
General anesthesia may be indicated in the performance of some procedures, in young or uncooperative children and in selected adult patients; however, a higher incidence of adverse reactions has been reported in these patients, and may be attributable to the inability of the patient to identify untoward symptoms, or to the hypotensive effect of anesthesia, which can reduce cardiac output and increase the duration of exposure to the contrast agent.
Seizure activity is rare (about 0.01%) on intravenous injection of ionic contrast media. However, in the higher doses used for CT in patients with brain metastases the incidence can be much higher (1% to 10%). In these patients prophylactic use of a small parenteral dose of diazepam is suggested immediately before injection when extra high dose CT regimens are employed.
In addition to the general precautions already described, excretory urography, angiography, and other uses also have hazards associated with the particular techniques employed. (See INDIVIDUAL INDICATIONS AND USAGE section.)
Information for Patients
Patients receiving injectable radiopaque diagnostic agents should be instructed to:
- Inform the physician if they are pregnant (see CLINICAL PHARMACOLOGY).
- Inform the physician if they are diabetic or if they have multiple myeloma, pheochromocytoma, homozygous sickle cell disease or known thyroid disorder (see WARNINGS—General).
- Inform the physician if they are allergic to any drugs, food, or if they have had any reactions to previous injections of dyes used for x-ray procedures (see PRECAUTIONS—General).
- Inform the physician about any other medications they are currently taking, including nonprescription drugs, before they are administered this drug.
Drug Interactions
Renal toxicity has been reported in a few patients with liver dysfunction who were given oral cholecystographic agents followed by urographic agents. Administration of intravascular urographic agents should therefore be postponed in any patient with a known or suspected hepatic or biliary disorder who has recently received a cholecystographic contrast agent.
Addition of an inotropic agent to contrast agents may produce a paradoxical depressant response which can be deleterious to the ischemic myocardium.
Diphenhydramine hydrochloride may cause precipitation when mixed in the same syringe with HYPAQUE-76.
Under certain circumstances (pH, temperature, concentrations, time), diatrizoate solutions are incompatible with promethazine hydrochloride, diphenhydramine hydrochloride, brompheniramine maleate, or papavarine hydrochloride solutions.
Do not prefill plastic syringes with HYPAQUE-76 for prolonged periods (i.e., for several hours or longer) before use.
Drug/Laboratory Test Interactions
If any of these studies, which might be affected by contrast media, are indicated, it is recommended that they be performed prior to administration of the contrast medium or two or more days afterwards.
Diatrizoate salts interfere with several laboratory urine and blood tests.
Blood Tests
Coagulation: Diatrizoate salts significantly inhibit all stages of coagulation. The fibrinogen concentration and Factors V, VII, and VIII are decreased. Prothrombin time and thromboplastin time are increased.
Platelet aggregation: High levels of plasma diatrizoates inhibit platelet aggregation.
Serum calcium: Diatrizoate salts may decrease serum calcium levels. However, this depletion of serum calcium may also be the result of the addition of chelating agents (edetate disodium) in the preparation of certain contrast media.
Red cell counts: Transitory decreases in red cell counts. Technetium-99m—RBC labeling interference.
Leukocyte counts: Decrease.
Urea nitrogen (BUN): Transitory increase (see CLINICAL PHARMACOLOGY).
Serum creatinine: Transitory increase.
Urine Tests
Contrast media which are excreted in the urine may interfere with some laboratory determinations, eg, proteinuria, specific gravity, osmolality, or bacterial cultures.
Thyroid Function Tests
Protein-bound iodine (PBI) and total serum organic iodine: Transient increase of both tests following urography have been noticed. The results of PBI and radioactive iodine uptake studies which depend on iodine estimations will not accurately reflect thyroid function for up to 16 days following administration of iodinated urographic media. However, thyroid function tests not depending on iodine estimations, eg, T3 resin uptake or free thyroxine assays, are not affected.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed in order to evaluate carcinogenic potential, mutagenesis, or whether HYPAQUE-76 can affect fertility in males or females.
Pregnancy Category C
Animal reproduction studies have not been conducted with HYPAQUE-76. It is also not known whether HYPAQUE-76 can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. HYPAQUE-76 should be given to a pregnant woman only if clearly needed.
Labor and Delivery
It is not known whether use of these contrast agents during labor or delivery has immediate or delayed adverse effects on the fetus, prolongs the duration of labor or increases the likelihood that forceps delivery or other obstetrical intervention or resuscitation of the newborn will be necessary.
Nursing Mothers
Diatrizoate salts are excreted unchanged in human milk. Because of the potential adverse reactions, although it has not been established that serious adverse reactions occur in nursing infants, caution should be exercised when these contrast media are administered to a nursing woman.
Pediatric Use
Infants and small children should not have any fluid restriction prior to excretory urography or any other procedures (see PRECAUTIONS—General). Guidelines for pediatric dosages are presented in DOSAGE AND ADMINISTRATION—General.
ADVERSE REACTIONS—General
Approximately 95 percent of adverse reactions accompanying the intravascular use of diatrizoate salts are of mild to moderate severity. However, life-threatening reactions and fatalities, mostly of cardiovascular origin, have occurred.
Adverse reactions to injectable contrast media fall into two categories: chemotoxic reactions and idiosyncratic reactions.
Chemotoxic reactions result from the physicochemical properties of the contrast media, the dose, and the speed of injection. All hemodynamic disturbances and injuries to organs or vessels perfused by the contrast medium are included in this category.
Idiosyncratic reactions include all other reactions. They occur more frequently in patients 20 to 40 years old. Idiosyncratic reactions may or may not be dependent on the amount of dose injected, the speed of injection, the mode of injection, and the radiographic procedure. Idiosyncratic reactions are subdivided into minor, intermediate, and severe. The minor reactions are self-limited and of short duration; the severe reactions are life-threatening and treatment is urgent and mandatory.
The reported incidence of adverse reactions to contrast media in patients with a history of allergy are twice that of the general population. Patients with a history of previous reactions to a contrast medium are three times more susceptible than other patients. However, sensitivity to contrast media does not appear to increase with repeated examinations.
Most adverse reactions to injectable contrast media appear within one to three minutes after the start of injection, but delayed reactions may occur.
Adverse reactions are grouped by organ system and listed below by decreasing order of occurrence and with an approximate incidence of occurrence. Significantly more severe reactions are listed before the other reactions regardless of frequency.
Greater Than 1 in 100 Patients
Body as a Whole: Reported incidences of death range from 6.6 per 1 million (0.00066 percent) to 1 in 10,000 patients (0.01 percent). Most deaths occur during injection or 5 to 10 minutes later, the main feature being cardiac arrest with cardiovascular disease as the main aggravating factor.
Isolated reports of hypotensive collapse and shock following urography are found in the literature. The incidence of shock is estimated to occur in 1 out of 20,000 (0.005 percent) patients.
Cardiovascular System: The most frequent adverse reaction to diatrizoate salts is vasodilation (feeling of warmth). The estimated incidence is 49 percent.
Digestive System: Nausea 6 percent, vomiting 3 percent.
Nervous System: Paresthesia 6 percent, dizziness 5 percent.
Respiratory System: Rhinitis 1 percent, increased cough 2 percent.
Skin and Appendages: Urticaria 1 percent.
Pain at the injection site is estimated to occur in about 12 percent of the patients undergoing urography. Pain is usually due to extravasation.
Painful hot erythematous swelling above the venipuncture site was estimated to occur in more than one percent of the patients undergoing phlebography.
Special Senses: Perversion of taste 11 percent.
Urogenital System: Osmotic nephrosis of the proximal tubular cells is estimated to occur in 23 percent of patients following excretory urography.
Less Than 1 in 100 Patients
Other infrequently reported reactions without accompanying incidence rates are listed below, grouped by organ system.
Body as a Whole: Malaria relapse, uremia, high creatinine and BUN (see PRECAUTIONS—General, Drug/Laboratory Test Interactions), thrombocytopenia, leukopenia, and anemia.
Cardiovascular System: Cerebral hematomas, hemodynamic disturbances, sinus bradycardia, transient electrocardiographic abnormalities, ventricular fibrillation, petechiae, chest pain, and cardiac arrest.
Digestive System: Severe unilateral or bilateral swelling of the parotid and submaxillary glands.
Nervous System: Convulsions, paralysis, and coma. (See PRECAUTIONS—General.)
Respiratory System: Asthma, dyspnea, laryngeal edema, pulmonary edema, and bronchospasm.
Skin and Appendages: Extravasation necrosis, urticaria with or without pruritus, mucocutaneous edema, and angioneurotic edema.
Special Senses: Bilateral ocular irritation, lacrimation, itching, conjunctival chemosis, infection, and conjunctivitis.
Urogenital: Renal failure, pain.
OVERDOSAGE
At dosage levels of diatrizoate sodium above a level containing 45 g of iodine, the incidence of unpleasant side effects increases. At total dosage equivalent to 80 gI or 90 gI administered over a short period of time (e.g., 30 minutes), clinical signs of systemic intolerance appear (mostly related to hyperosmolar effects) and are manifest as tremors, irritability, and tachycardia. Above these maximal tolerated dosage levels in otherwise healthy adults, an increasing incidence and severity of dyspnea and pulmonary edema should be expected.
Four cases of overdosage in infants, during urography, are reported. Three of the infants died within 19 hours of the injection. The overdose ranged from slightly above the recommended pediatric dosage to a dose exceeding 19 g/kg. The symptoms of overdosage appeared between 10 minutes to several hours after injection of the contrast medium. Adverse effects were life-threatening, affecting mainly the pulmonary and cardiovascular systems. The symptoms included: cyanosis, bradycardia, acidosis, pulmonary hemorrhage, convulsions, coma, and cardiac arrest. All infants showed a poor visualization of the kidneys and a diffuse opacification of all the tissues and vasculature. Autopsy findings showed acute pulmonary damage and/or edema of subcutaneous tissues. Treatment of an overdose of injectable radiopaque contrast media is directed toward the support of all vital functions, and prompt institution of symptomatic therapy.
The acute intravenous LD50 of diatrizoate salts in mice is equivalent in iodine content of 5.3 gI/kg to 8.0 gI/kg and seems to be directly proportioned to the rate of injection.
Diatrizoate salts are dialyzable.
DOSAGE AND ADMINISTRATION—General
Preparation of the patient will vary with preference of the radiologist and the type of radiological procedure performed. Specific radiographic procedures used will depend on the state of the patient and the diagnostic indications. Individual dose should be tailored according to age, body size, and indication for examination. (See INDIVIDUAL INDICATIONS AND USAGE section for specific Dosage and Administration.)
Solutions of radiopaque diagnostic agents for intravascular use should be at body temperature when injected and may need to be warmed before use. In the event that crystallization occurs, the solution may be clarified by placing the vial in a water bath at 40°C to 50°C and shaking it gently for two to three minutes or until the solids redissolve. If the particles still persist, do not use this vial but discard it. The solution may be autoclaved once.
The solution should be stored at room temperature and protected from strong light and any unused portion remaining in the container should be discarded.
Dilution and withdrawal of the contrast agents should be accomplished under aseptic conditions with sterile syringes.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Avoid contaminating catheters, syringes, needles, and contrast media with glove powder or cotton fibers.
Pediatric Dosage
Pediatric doses of injectable radiopaque diagnostic agents are generally determined on a weight basis and should be calculated for each patient individually. (See INDIVIDUAL INDICATIONS AND USAGE section.)
Drug Incompatibilities
Diatrizoate salts are incompatible in vitro with some antihistamines and many other drugs. It is believed that one of the chief causes of in vitro incompatibility is an alteration of pH. Turbidity of solutions of intravascular contrast medium occurs between pH 2.5 and 4.1. Another cause is chemical interaction; therefore, other pharmaceuticals should not be mixed with contrast agents in the same syringe.
INDIVIDUAL INDICATIONS AND USAGE
THE FOLLOWING SECTIONS FOR INDIVIDUAL INDICATIONS AND USAGE CONTAIN CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, ADVERSE REACTIONS, AND DOSAGE AND ADMINISTRATION SECTIONS RELATED TO THE SPECIFIC PROCEDURES. HOWEVER, IT SHOULD BE UNDERSTOOD THAT THE INFORMATION IN THE GENERAL SECTIONS IS ALSO LIKELY TO APPLY TO ALL OF THESE SPECIFIC USES.
Hydration: With the possible exception of urography, patients should be fully hydrated prior to the following procedures.
EXCRETORY UROGRAPHY
Diatrizoate salts are used in small, medium, and large dose urography. Visualization of the urinary tract can be achieved by either direct intravenous bolus injection, intravenous drip infusion, or incidentally following intra-arterial procedures. Visualization of the urinary tract is delayed in infants less than 1 month old, and in patients with urinary tract obstruction (see CLINICAL PHARMACOLOGY).
Nephrotomography and "Adequate" or High Dose Urography
HYPAQUE-76 solution may be used for excretory urography in selected patients. It may be used when prior urography has failed to provide diagnostic contrast and for preliminary excretory tract study to detect obstruction in azotemic patients or to avoid retrograde instrumentation. It may also be used as a high dosage medium to intensify and prolong the nephrographic effect when the prime purpose is examination of the renal parenchyma, especially with tomography. When combined with the urea "washout" technique, HYPAQUE-76 provides the necessary increased pyelographic contrast and diuresis required for screening or special examination of patients with suspected renal hypertension.
Contraindication
Urography is contraindicated in patients with anuria.
Precautions
Although azotemia is not considered a contraindication, care is required in patients with advanced renal failure. The usual preparatory dehydration should be omitted, and urinary output should be observed for one to two days in these patients. Adequate visualization may be difficult or impossible to attain in patients with severely impaired renal and/or hepatic function. Use with extreme caution in patients with concomitant hepatorenal disease.
When HYPAQUE-76 is used with a urea "washout" procedure, the patient should also be observed for a few hours to detect signs of undue dehydration caused by increased diuresis induced by both the medium and the urea. Ingestion of water may be required for rehydration.
In myelomatosis, urography should only be performed with caution. If a weak protein-binding agent such as a diatrizoate is used for the procedure, it is essential to omit preparatory dehydration, administer fluids, and attempt to alkalinize the urine.
Preparatory Dehydration: Preparatory dehydration is dangerous in infants, young children, the elderly, and azotemic patients (especially those with polyuria, oliguria, diabetes, advanced vascular disease, or preexisting dehydration). The undesirable dehydration in these patients may be accentuated by the osmotic diuretic action of the medium.
Dehydration may improve image quality in patients with adequate renal function particularly if a low dose is used. Dehydration, however, will not improve contrast quality in patients with substantial renal insufficiencies and will increase risk of contrast induced renal damage. Dehydration in these patients is therefore contraindicated.
Side Effects
Side effects following this relatively high dose urography are usually mild and transitory and do not appear to occur more frequently or severely than those induced by "standard dose" urography. Nausea, facial flushing, and emesis are not uncommon reactions. (See also ADVERSE REACTIONS—General.)
Dosage and Administration
The usual intravenous dose for adults is 20 mL, with a range of 20 mL to 40 mL. Children require less in proportion to weight: Under 6 months of age—4 mL; 6 to 12 months—6 mL; 1 to 2 years—8 mL; 2 to 5 years—10 mL; 5 to 7 years—12 mL; 8 to 10 years—14 mL; 11 to 15 years—16 mL.
ANGIOGRAPHY—General
Diatrizoate salts are used for radiographic studies throughout the cardiovascular system.
Intravascular radiopaque diagnostic agents of high concentration are not recommended for cerebral or spinal angiography (see CONTRAINDICATIONS—General), and contrast agents with the lowest compatible viscosity and higher concentration of iodine (310 mg/mL to 480 mg/mL of bound iodine) must be used for angiocardiography. Contrast media approaching serum ionic content and osmolality have less potential for deleterious effects on the myocardium (see PRECAUTIONS—General, Drug Interactions).
Addition of chelating agents may contribute to toxicity in coronary angiography, and the sodium content of angiographic agents used in coronary arteriography is of crucial importance.
AORTOGRAPHY
HYPAQUE-76 solution may be injected by commonly accepted techniques, such as translumbar, retrograde catheter, retrograde pressure injection (by cannula) or antegrade (brachial) catheter, for examination of the aorta and its major branches.
Warnings
During aortography by the translumbar technique, extreme care is advised to avoid inadvertent intrathecal injection since the injection of even small amounts (5 mL to 7 mL) of the contrast medium may cause convulsions, permanent sequelae, or fatality. Should the accident occur, the patient should be placed upright to confine the hyperbaric solution to a low level, anesthesia may be required to control convulsions, and if there is evidence of a large dose having been administered, a careful cerebrospinal fluid exchange-washout should be considered.
Pheochromocytoma: Administration of angiographic media to patients known or suspected to have pheochromocytoma can cause dangerous changes in blood pressure. A minimum dose should be injected. The blood pressure should be carefully monitored and measures for controlling major fluctuations should be available.
Precautions
The presence of a vigorous pulsatile flow should be established before using a catheter or pressure injection technique. A small "pilot" dose (about 2 mL) should be administered to locate the exact site of needle or catheter tip to help prevent injection of the main dose into a branch of the aorta or intramurally. In the translumbar technique, severe pain during injection may indicate intramural placement and abdominal or back pain afterwards may indicate hemorrhage from the injection site. Following catheter procedures, gentle pressure hemostasis for 5 to 10 minutes is advised, followed by observation for 30 to 60 minutes and immobilization of the limb for several hours to prevent hemorrhage from the site of arterial puncture.
Under conditions of slowed aortic circulation there is an increased likelihood of aortography causing muscle spasm. Occasional serious neurologic complications, including paraplegia, have also been reported in patients with aortic-iliac or even femoral artery bed obstruction, abdominal compression, hypotension, hypertension, spinal anesthesia, injection of vasopressors to increase contrast, and low injection sites (L2-3). In these patients the concentration, dose, and number of repeat injections of the medium should be maintained at a minimum with appropriate intervals between injections. The position of the patient and catheter tip should be carefully evaluated.
Side Effects
The only adverse reaction expected is a mild burning or painful sensation on injection. Unusual reactions can occur, as in angiocardiography. An abrupt hypertensive episode can occur following entry of the medium into the renal artery in patients with pheochromocytoma. Mesenteric necrosis, acute pancreatitis, renal shutdown (usually transitory), and neurologic complications have been reported following inadvertent injection of a large part of the aortic dose into a branch of the aorta. Entry of the large aortic dose into the renal artery can cause, even in the absence of symptoms, albuminuria, cylindruria, hematuria, and an elevated BUN. Rapid and complete return of function usually follows.
Additional procedural reactions include injury to the aorta and neighboring organs, pleural puncture, renal damage including infarction and acute tubular necrosis with oliguria and anuria, accidental selective filling of the right renal artery during the translumbar procedure in the presence of preexistent renal disease, and retroperitoneal hemorrhage from the translumbar approach.
Dosage and Administration
Adult: The usual adult dose as a single injection is 15 mL to 40 mL, repeated if indicated, to a total of 160 mL.
Pediatric: The usual single pediatric dose ranges from 0.3 mL/kg to 0.9 mL/kg. The total dose should not exceed 1 mL/kg.
ANGIOCARDIOGRAPHY
Pharmacology-Hemodynamic Changes
Due to its physical characteristics (chiefly tonicity and viscosity) and the volume administered, HYPAQUE-76 may cause a number of transitory hemodynamic changes. When the medium is ejected from the left ventricle or introduced at the root of the ascending aorta, a brief (three seconds) hypertensive response is usually induced, followed immediately by a decrease in aortic and peripheral blood pressures below normal levels, lasting for at least two minutes. This hypotensive phase may be followed by a 15- to 20-minute period of fluctuating blood pressure.
Clinical doses (up to 1 mL per kg) injected into the vena cava or right heart outflow tract usually cause an irregular rise in right ventricular blood pressure, a slight increase in pulmonary artery blood pressure, and delayed signs of peripheral hypotension.
Other changes reported clinically include an increase in cardiac output and atrial pressure, a decrease in myocardial contractile force, and at the peak of postinjection hypotension, a marked rise in aortic and carotid blood flow, and elevation of central venous pressure. At dosage levels used in angiocardiography, the hematocrit and hemoglobin level may fall about 10 to 15 percent and serum osmolality may rise 10 to 12 percent. Blood carbon dioxide, pH, and BUN levels may fall. These changes commence immediately after injection, reach a maximum in two to five minutes, and return to normal values in 10 to 15 minutes. However, after the initial rise, plasma volume may decrease and continue to fall below control levels, even beyond 30 minutes, probably due to diuresis. If repeat injections are made in rapid succession these changes are likely to be more pronounced. (See also Dosage and Administration.) HYPAQUE-76 is not metabolized. It is eliminated unchanged, rapidly and completely in the urine by glomerular filtration.
Precautions
During administration of large doses of HYPAQUE-76 continuous monitoring of vital signs is desirable. Caution is advised in the administration of large doses to patients with incipient heart failure because of the possibility of aggravation of the preexisting condition. Hypotension should be corrected promptly since it may induce serious arrhythmias.
Because of the hemodynamic changes which may occur on injection into the right heart outflow tract, special care, especially regarding dosage, should be observed in patients with right ventricular failure, pulmonary hypertension, or obliterated pulmonary vascular beds.
Precautions in Infants
Apnea, bradycardia and other arrhythmias, cerebral effects (lethargy and depression), and a tendency to acidosis are more likely to occur in cyanotic infants. It is desirable that vital signs be monitored on an intensive care basis afterwards to detect delayed adverse effects (arrhythmia, electrolyte and hemodynamic disturbances). Infants are more likely than adults to respond with convulsions, particularly after repeated injections. Unlike in the adult, the amount of the total dosage in young infants is of particular importance. (See Dosage and Administration.)
Side Effects
See ADVERSE REACTIONS—General.
Dosage and Administration
The individual dose is determined by the size of the structure to be visualized, the anticipated degree of hemodilution, and valvular competence. Weight is a minor consideration in adults. The size of each individual dose is a more important consideration than the total dosage used. When large individual doses are administered, as for contrast in the cardiac chambers and thoracic aorta, it has been suggested that 20 minutes be permitted to elapse between each injection to allow for subsidence of hemodynamic disturbances.
Ventriculography
Adult: The usual adult dose in a single injection is 45 mL, with a range of 40 mL to 50 mL. This may be repeated as necessary. However, when combined with selective coronary arteriography, the total dose should not exceed 225 mL.
Pediatric: The usual single pediatric dose is 0.2 mL/kg to 0.3 mL/kg. The total dose should not exceed 50 mL.
Pulmonary Angiography or Arch Study Used Alone
Adult: The usual single adult dose is 30 mL, with a range of 10 mL to 56 mL.
Pediatric: The pediatric dose ranges from 0.3 mL/kg to 0.9 mL/kg. The total dose should not exceed 1 mL/kg.
Combined Angiocardiographic Procedures
Multiple Procedures
With continuing advances in radiologic techniques and the development of more sophisticated equipment as well as more versatile and reliable radiopaque media, it is possible to examine multiple vascular systems and target organs during a single radiographic examination of the patient.
Multiple procedures of selective angiography require multiple injections of the contrast medium into several specific target organs and result in a greater dosage. Large doses of HYPAQUE-76 were well tolerated in multiple injections and multiple procedures of angiography.
See general sections for Contraindications, Warnings, Precautions, Adverse Reactions, and Dosage and Administration recommendations as well as those sections pertinent to the specific procedures.
Adult: The maximum total dose for multiple procedures in adult use should not exceed 225 mL.
Pediatric: When multiple procedures are required in pediatric use, the total dose administered should be maintained below 4 mL/kg, or especially in young infants below 3 mL/kg.
Selective Coronary Arteriography
Adult: The usual single adult dose for right or left coronary arteriography is 8 mL (range 4 mL to 10 mL) repeated as required up to a total dose of 120 mL for coronary arteriography alone.
PERIPHERAL ANGIOGRAPHY
The use of HYPAQUE-76 is sometimes preferred over less concentrated media in selected cases for femoral and brachio-axillary arteriography in adults.
Precautions
Pulsation should be present in the artery to be injected. In thromboangiitis obliterans, or ascending infection associated with severe ischemia, angiography should be performed with extreme caution, if at all.
Side Effects
Pain or a burning sensation with some spasm may occur, and is more marked in patients with arterial insufficiency. Therefore, the procedure is more satisfactorily performed under general or, for the lower extremity, spinal anesthesia.
Technical complications have included hemorrhage from the puncture site, and brachial plexus palsy following axillary artery injections.
Arterial thrombosis, displacement of arterial plaques, and ipsilateral venous thrombosis are very rare complications.
Dosage and Administration
Arteriography (aorto-iliac runoff and peripheral)
Adult: The single adult dose for femoral arteriography varies from 20 mL to 40 mL, depending on the site of placement, i.e., aorta-iliac runoff, iliofemoral, femoral. For the upper limb, 20 mL to 40 mL is usually sufficient. These doses may be repeated up to three times.
VENOGRAPHY
Dosage and Administration
Central Venography
Adult: For central venography (inferior or superior vena cava), the total adult dose is 40 mL to 50 mL, repeated up to two times.
Renal Venography
Adult: The usual single adult dose is 20 mL to 40 mL.
Peripheral Venography
Adult: The usual single adult dose is 40 mL with a range of 40 mL to 60 mL.
SELECTIVE RENAL ARTERIOGRAPHY
Dosage and Administration
Adult: The usual single adult dose is 8 mL, repeated up to three times. The dose ranges from 5 mL to 10 mL.
SELECTIVE VISCERAL ARTERIOGRAPHY
Dosage and Administration
Celiac Axis: The usual adult dose ranges from 30 mL to 40 mL with subselective arteriography of its branches, e.g., hepatic, usual dose of 25 mL with a range of 15 mL to 30 mL; mesenteric, usual dose of 30 mL with a range of 20 mL to 40 mL; splenic, usual dose of 20 mL with a range of 20 mL to 50 mL.
Superior Mesenteric: The usual single adult dose is 30 mL with a range of 20 mL to 40 mL. The maximum total dose should not exceed 175 mL.
INTRAVENOUS DIGITAL ARTERIOGRAPHY
Arteriograms of diagnostic quality can be obtained following the intravenous administration of HYPAQUE-76 employing digital subtraction and computer imaging enhancement equipment. The intravenous route of administration using these techniques has the advantage of being less invasive than the corresponding selective catheter placement of the medium. The dose is administered into a peripheral vein usually by mechanical injection although sometimes by rapid manual injection. The technique has been used most frequently to visualize the ventricles, the aorta and most of its larger branches including carotid, cerebral, vertebral, renal, celiac, mesenterics, and the major peripheral vessels of the limbs.
Precautions
Since the dose is usually administered mechanically under high pressure, rupture of smaller peripheral veins has occurred. It has been suggested that this can be avoided by using an intravenous catheter threaded proximally beyond larger tributaries or in the case of the antecubital vein, into the superior vena cava. Sometimes the femoral vein is used.
Dosage and Administration
The usual dose of HYPAQUE-76 per injection by the intravenous digital technique is 30 mL to 60 mL with a range of 0.5 mL/kg to 1 mL/kg administered as a bolus 7.5 mL/second to 30 mL/second using a pressure injector. The dose and rate of injection will depend primarily on the type of equipment and technique used, with first exposures made on calculated circulation time.
CONTRAST ENHANCEMENT OF COMPUTED TOMOGRAPHIC IMAGING
Injectable radiopaque contrast media may be used to refine diagnostic precision in areas of the brain which may not otherwise have been satisfactorily visualized.
Tumors: Radiopaque diagnostic agents may be useful to investigate the presence and extent of certain malignancies such as: gliomas including malignant gliomas, glioblastomas, astrocytomas, oligodendrogliomas and gangliomas, ependymomas, medulloblastomas, meningiomas, neuromas, pinealomas, pituitary adenomas, craniopharyngiomas, germinomas, and metastatic lesions. The usefulness of contrast enhancement for the investigation of the retrobulbar space and in cases of low grade or infiltrative glioma has not been demonstrated.
In calcified lesions, there is less likelihood of enhancement. Following therapy, tumors may show decreased or no enhancement.
Nonneoplastic Conditions of the Brain: The use of HYPAQUE-76 may be beneficial in the enhancement of images of lesions not due to neoplasms. Cerebral infarctions of recent onset may be better visualized with the contrast enhancement, while some infarctions are obscured if a contrast medium is used. The use of HYPAQUE-76 improved the contrast enhancement in approximately 60 percent of cerebral infarctions studied from one week to four weeks from the onset of symptoms.
Sites of active infection also will produce contrast enhancement following contrast medium administration.
Arteriovenous malformations and aneurysms will show contrast enhancement. In the case of these vascular lesions, the enhancement is probably dependent on the iodine content of the circulating blood pool.
Hematomas and intraparenchymal bleeders seldom demonstrate any contrast enhancement. However, in cases of intraparenchymal clot, for which there is no obvious clinical explanation, contrast medium administration may be helpful in ruling out the possibility of associated arteriovenous malformation.
The opacification of the inferior vermis following contrast medium administration has resulted in false-positive diagnoses in a number of normal studies.
Dosage and Administration
The suggested dose is 50 mL to 125 mL by intravenous administration; scanning may be performed immediately after completion of administration. Doses for children should be proportionately less, depending on age and weight.
CONTRAST ENHANCEMENT OF COMPUTED TOMOGRAPHIC IMAGING
HYPAQUE-76, brand of diatrizoate meglumine and diatrizoate sodium injection, may be used for enhancement of computed tomographic scans performed for detection and evaluation of lesions in the liver, pancreas, kidneys, aorta, mediastinum, abdominal cavity, pelvis, and retroperitoneal space.
Enhancement of computed tomography with HYPAQUE-76 may be of benefit in establishing diagnoses of certain lesions in these sites with greater assurance than is possible with CT alone, and in supplying additional features of the lesions (e.g., hepatic abscess delineation prior to percutaneous drainage). In other cases, the contrast agent may allow visualization of lesions not seen with CT alone (e.g., tumor extension), or may help to define suspicious lesions seen with unenhanced CT (e.g., pancreatic cyst).
Contrast enhancement appears to be greatest within 60 to 90 seconds after bolus administration of the contrast agent. Therefore, utilization of a continuous scanning technique (dynamic CT scanning) may improve enhancement and diagnostic assessment of tumor and other lesions such as an abscess, occasionally revealing unsuspected or more extensive disease. For example, a cyst may be distinguished from a vascularized solid lesion when precontrast and enhanced scans are compared; the nonperfused mass shows unchanged x-ray absorption (CT number). A vascularized lesion is characterized by an increase in CT number in the few minutes after a bolus of intravascular contrast agent; it may be malignant, benign or normal tissue, but would probably not be a cyst, hematoma, or other nonvascular lesion.
Because unenhanced scanning may provide adequate diagnostic information in the individual patient, the decision to employ contrast enhancement, which may be associated with risk and increased radiation exposure, should be based upon a careful evaluation of clinical, other radiological and unenhanced CT findings.
Dosage and Administration
The suggested dose is 50 mL to 125 mL by rapid intravenous bolus administration; scanning may be performed immediately after completion of administration. Doses for children should be proportionately less, depending on age and weight.
HOW SUPPLIED
Vials of 50 mL, rubber stoppered, box of 25 (NDC 0407-0776-04)
Calibrated bottles of 200 mL, rubber stoppered, with hangers, box of 10 (NDC 0407-0778-02)
Distributed by Amersham Health Inc.
Princeton, NJ 08540
Printed In USA
HNC-9D
HYPAQUEDiatrizoate Meglumine and Diatrizoate Sodium INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||
HYPAQUEDiatrizoate Meglumine and Diatrizoate Sodium INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||