HylatopicPlus
Hylatopic Plus CreamNON-COMEDOGENIC PARABEN FREE
FULL PRESCRIBING INFORMATION: CONTENTS*
- INDICATIONS FOR USE
- HYLATOPICPLUS CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS AND OBSERVATIONS
- INSTRUCTIONS FOR USE
- INGREDIENTS
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
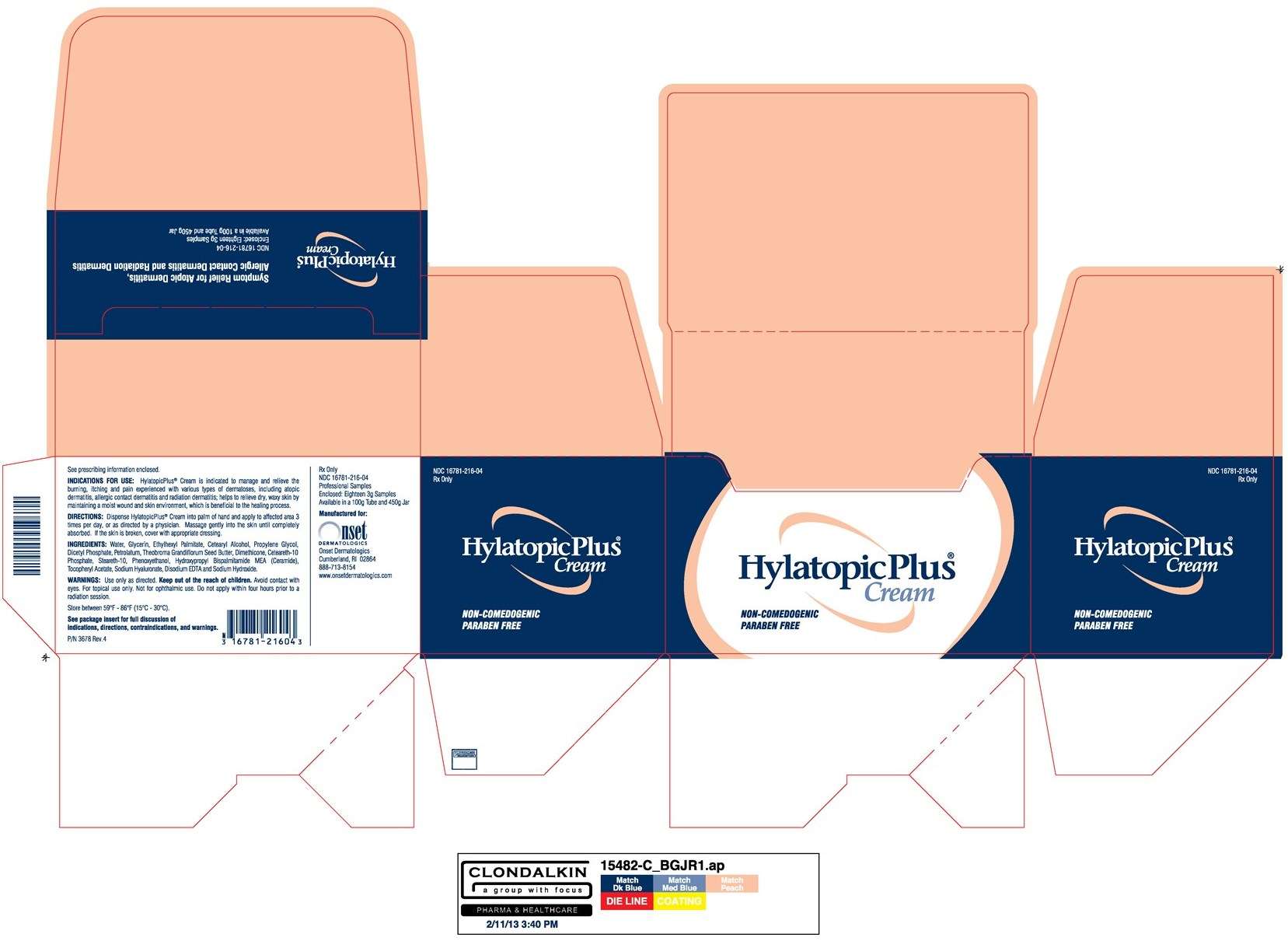
INDICATIONS FOR USE
Under the supervision of a healthcare professional, HylatopicPlus® Cream is indicated to manage and relieve the burning, itching and pain experienced with various types of dermatoses, including atopic dermatitis, allergic contact dermatitis and radiation dermatitis. HylatopicPlus® Cream also helps to relieve dry, waxy skin by maintaining a moist wound & skin environment, which is beneficial to the healing process.
HYLATOPICPLUS CONTRAINDICATIONS
HylatopicPlus® Cream is contraindicated in persons with a known hypersensitivity to any of the components of the formulation.
WARNINGS
Use only as directed. Keep out of the reach of children. Avoid contact with eyes. For topical use only. Not for ophthalmic use. Do not apply within four hours prior to a radiation session.
PRECAUTIONS AND OBSERVATIONS
- HylatopicPlus® Cream does not contain a sunscreen and should not be used prior to extended exposure to the sun.
- If clinical signs of infection are present, appropriate treatment should be initiated; use of HylatopicPlus® Cream may be continued during the anti-infective therapy.
- If the condition does not improve within 10-14 days, consult a physician.
- HylatopicPlus® Cream may dissolve fuchsin when this dye is used to define the margins of the radiation fields to be treated.
INSTRUCTIONS FOR USE
Dispense HylatopicPlus® Cream into palm of hand and apply to affected area 3 times per day, or as directed by a physician.Massage gently into the skin until completely absorbed. If the skin is broken, cover with appropriate dressing.
INGREDIENTS
Water, Glycerin, Ethylhexyl Palmitate, Cetearyl Alcohol, Propylene Glycol, Dicetyl Phosphate, Petrolatum, Theobroma Grandiflorum Seed Butter, Dimethicone, Ceteareth-10 Phosphate, Steareth-10, Phenoxyethanol, Hydroxypropyl Bispalmitamide MEA (Ceramide), Tocopheryl Acetate, Sodium Hyaluronate, Disodium EDTA and Sodium Hydroxide.
HOW SUPPLIED
HylatopicPlus® Cream is available in 3g professional sample tubes (NDC 16781-216-04),100g commercial tubes (NDC 16781-216-96) and 450g commercial jars (NDC 16781-216-16).
Caution: Federal law restricts this device to sale by or on the order of a physician or other licensed health care practitioner.
Store between 59°F - 86°F (15°C - 30°C).
Manufactured for:
Onset Dermatologics
Cumberland, RI 02864
(888)
713-8154
www.onsetdermatologics.com

Rx Only
For Topical
Dermatological and
External Use
Only

Symptom relief for atopic dermatitis, allergic contact dermatitis, and radiation dermatitis
P/N 2623 Rev. 4
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 450 Gram Jar Carton
NDC 16781-216-16
Rx Only
HylatopicPlus Cream
Symptom Relief for Atopic Dermatitis,
Allergic Contact Dermatitis and Radiation Dermatitis
Net Weight 450g (15.9 oz)

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 100 Gram Tube Carton
NDC 16781-216-96
HylatopicPlus Cream
Rx Only
Net Weight 100g

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 3 Gram Carton
NDC 16781-216-04
HylatopicPlus
Cream
Rx Only Device
Professional Sample
Not for Sale
Net Weight 3g
HylatopicPlus CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||