Hydrocodone Bitartrate and Acetaminophen
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- HYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
HYDROCODONE BITARTRATE AND ACETAMINOPHEN DESCRIPTION
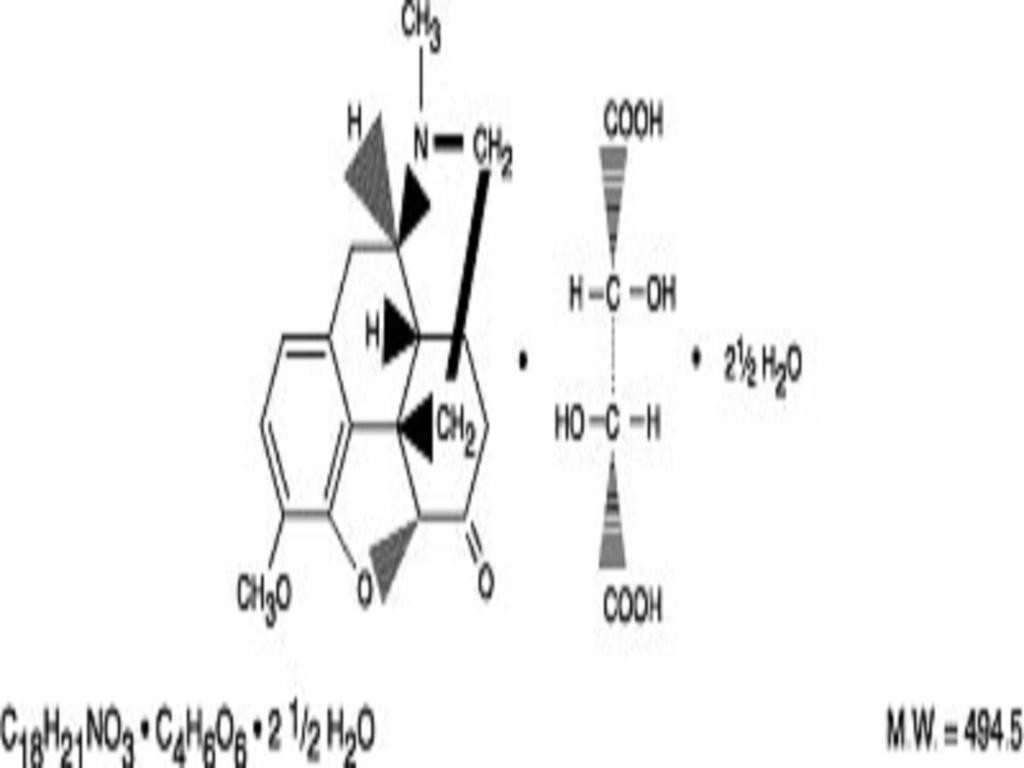
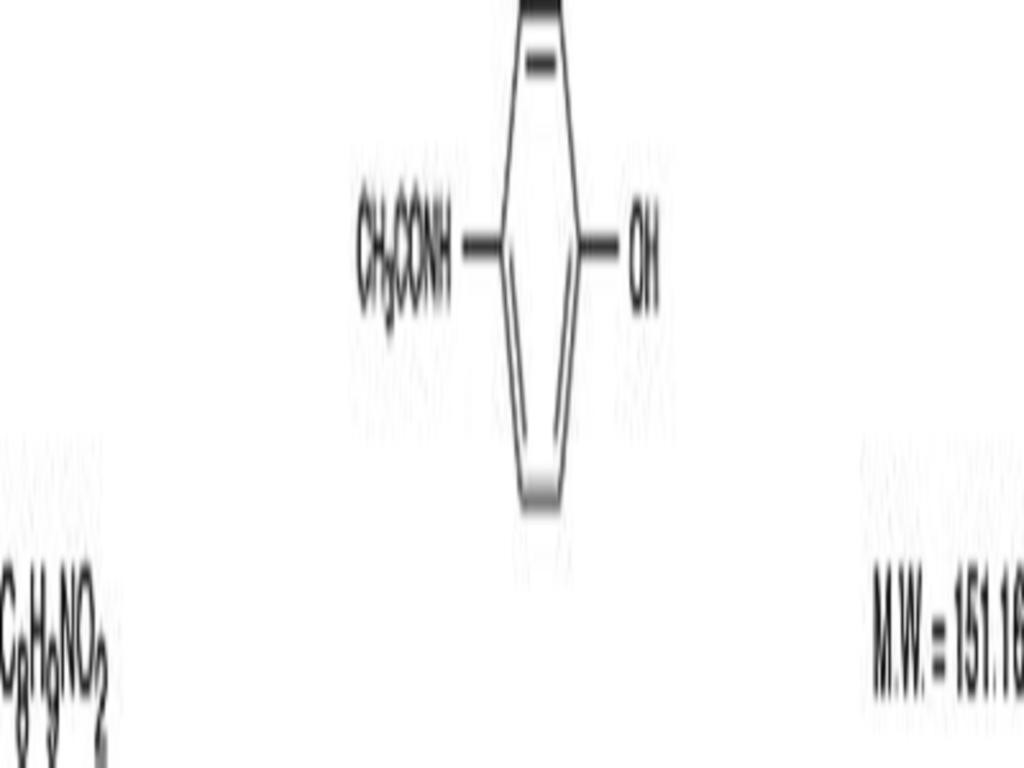
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 2.5 mg/500 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 5 mg/325 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 5 mg/500 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/325 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/500 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/650 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/750 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/325 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/500 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/650 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/660 mg
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
Pharmacokinetics:Hydrocodone:
OVERDOSAGE
Acetaminophen:
OVERDOSAGE
INDICATIONS & USAGE
HYDROCODONE BITARTRATE AND ACETAMINOPHEN CONTRAINDICATIONS
WARNINGS
Respiratory Depression:Head Injury and Increased Intracranial Pressure:
Acute Abdominal Conditions:
Misuse, Abuse, and Diversion of Opioids:
DRUG ABUSE AND DEPENDENCE
PRECAUTIONS
General:Special Risk Patients:
Cough Reflex:
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of Fertility:PREGNANCY
Teratogenic Effects:Pregnancy Category C:
Nonteratogenic Effects:
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
HYDROCODONE BITARTRATE AND ACETAMINOPHEN ADVERSE REACTIONS
OVERDOSAGE
OVERDOSAGE
DRUG ABUSE AND DEPENDENCE
Misuse, Abuse, and Diversion of Opioids:OVERDOSAGE
Signs and Symptoms:
Hydrocodone:
Acetaminophen:
Treatment:
DOSAGE & ADMINISTRATION
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 2.5 mg/500 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 5 mg/325 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 5 mg/500 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/325 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/500 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/650 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/750 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/325 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/500 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/650 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/660 mg
HOW SUPPLIED
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 2.5 mg/500 mgHydrocodone Bitartrate and Acetaminophen Tablets, USP 5 mg/325 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 5 mg/500 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/325 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/500 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/650 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 7.5 mg/750 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/325 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/500 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/650 mg
Hydrocodone Bitartrate and Acetaminophen Tablets, USP 10 mg/660 mg
STORAGE AND HANDLING
INACTIVE INGREDIENT
SILICON DIOXIDECROSCARMELLOSE SODIUM
CROSPOVIDONE
FD&C RED NO. 3
MAGNESIUM STEARATE
CELLULOSE, MICROCRYSTALLINE
POVIDONE
STARCH, CORN
STEARIC ACID
SUCROSE
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Hydrocodone Bitartrate and AcetaminophenHydrocodone Bitartrate and Acetaminophen TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!