hydrochlorothiazide
NorthStar RxLLC
Alembic Limited
Hydrochlorothiazide Capsules, 12.5 mg Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- HYDROCHLOROTHIAZIDE INDICATIONS AND USAGE
- HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- HYDROCHLOROTHIAZIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
HYDROCHLOROTHIAZIDE DESCRIPTION
H78342
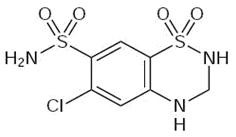
Inactive ingredients:
CLINICAL PHARMACOLOGY
Pharmacokinetics and Metabolism:
Pharmacodynamics:
Clinical Studies:
HYDROCHLOROTHIAZIDE INDICATIONS AND USAGE
Usage in Pregnancy:
HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
Hydrochlorothiazide capsules are contraindicated in patients with anuria. Hypersensitivity to this product or other sulfonamide derived drugs is also contraindicated.
WARNINGS
Diabetes and Hypoglycemia:
Renal Disease:
PRECAUTIONS
Electrolyte and Fluid Balance Status:
Hyperuricemia:
Impaired Hepatic Function:
Parathyroid Disease:
Drug Interactions
Alcohol, barbiturates, or narcotics
Antidiabetic drugs
Other antihypertensive drugs
Cholestyramine and colestipol resins
Corticosteroid, ACTH
Pressor amines (e.g., norepinephrine)
Skeletal muscle relaxants, nondepolarizing (e.g., tubocurarine)
Lithium
Non-steroidal anti-inflammatory drugs
Drug/Laboratory Test Interactions - PRECAUTIONS, General
Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitro Salmonella typhimurium in vivo Drosophila in vitro Aspergillus nidulans
Pregnancy
Teratogenic Effects
Pregnancy Category B:
Nonteratogenic Effects
Thiazides cross the placental barrier and appear in cord blood. There is a risk of fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
Nursing Mothers
Thiazides are excreted in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue hydrochlorothiazide, taking into account the importance of the drug to the mother.
Pediatric Use
Elderly Use
A greater blood pressure reduction and an increase in side effects may be observed in the elderly (i.e., >65 years) with hydrochlorothiazide. Starting treatment with the lowest available dose of hydrochlorothiazide (12.5 mg) is therefore recommended. If further titration is required, 12.5 mg increments should be utilized.
HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
Body as a whole:
Cardiovascular:
Digestive:
Hematologic:
Hypersensitivity:
Metabolic: PRECAUTIONS
Musculoskeletal:
Nervous System/Psychiatric:
Renal: WARNINGS
Skin:
Special Senses:
Urogenital:
OVERDOSAGE
50
HYDROCHLOROTHIAZIDE DOSAGE AND ADMINISTRATION
For Control of Hypertension: The adult initial dose of hydrochlorothiazide capsules is one capsule given once daily whether given alone or in combination with other antihypertensives. Total daily doses greater than 50 mg are not recommended.
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

hydrochlorothiazideHydrochlorothiazide CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||