Hydro 35
Quinnova Pharmaceuticals, Inc.
Hydrating Topical Foam
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDRO 35 DESCRIPTION
- CHEMICAL STRUCTURE
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- HYDRO 35 INDICATIONS AND USAGE
- HYDRO 35 CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- HYDRO 35 ADVERSE REACTIONS
- HYDRO 35 DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
- DISPLAY PANEL
- DISPLAY PANEL
FULL PRESCRIBING INFORMATION
DESCRIPTION
HYDRO 35 is a keratolytic emollient in a water and lipid based foam containing lactic acid which is a gentle, but potent, tissue softener for skin and nails. Each gram of HYDRO 35 contains Urea 35% as the active ingredient, and the following inactive ingredients: dimethicone, ethylparaben, glycerin, lactic acid, methylparaben, phenoxyethanol, polysorbate 20, povidone, propylene glycol, propylparaben, purified water, stearic acid, trolamine, and in propellants butane and propane.
CHEMICAL STRUCTURE
Urea has the following chemical structure:
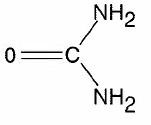
CLINICAL PHARMACOLOGY
Topically applied urea dissolves the intercellular matrix of the skin which results in enhanced shedding of scaly, dry skin and thus a softening of the hyperkeratotic areas of the skin.
Urea topically applied to the nail plate has a similar effect on the intercellular matrix of the nail plate.
PHARMACOKINETICS
The mechanism of action of topically applied urea is not yet known.
INDICATIONS AND USAGE
For enzymatic debridement and promotion of normal healing of surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris, or eschar. Topically applied urea is useful for the treatment of hyperkeratotic conditions such as dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, and dry, rough skin, as well as corns and calluses and damaged, ingrown and devitalized nails.
CONTRAINDICATIONS
Known hypersensitivity to any of the listed ingredients.
WARNINGS
HYDRO 35 is for external use only. It is not for ophthalmic, oral, anal or intravaginal use. Contact with eyes, lips and all mucous membranes should be avoided. HYDRO 35 should not be used by persons who have a known hypersensitivity to urea or any of the other listed ingredients.
PRECAUTIONS
HYDRO 35 should be used only as directed by a physician and should not be used to treat any condition other than that for which it is prescribed. If redness or irritation occurs, discontinue use and consult with prescribing physician.
Pregnancy (Category B) - Animal reproduction studies have not been performed with topically applied urea and it is not known whether HYDRO 35 can cause fetal harm when administered to a pregnant woman. Nevertheless, HYDRO 35 should be used by a pregnant woman only if necessary.
Nursing Mothers - It is not known whether topically applied urea is excreted in human milk. Due to the fact that many drugs are excreted in human milk, caution should be exercised by physicians when administering HYDRO 35 to nursing mothers.
KEEP THIS AND ALL OTHER MEDICATIONS OUT OF THE REACH OF CHILDREN.
ADVERSE REACTIONS
Transient stinging, burning, itching or irritation is possible.
DOSAGE AND ADMINISTRATION
Unless otherwise directed by a prescribing physician,
HYDRO 35 should be applied to affected area twice a day.
HYDRO 35 should be rubbed into the skin until it is completely absorbed.
HOW SUPPLIED
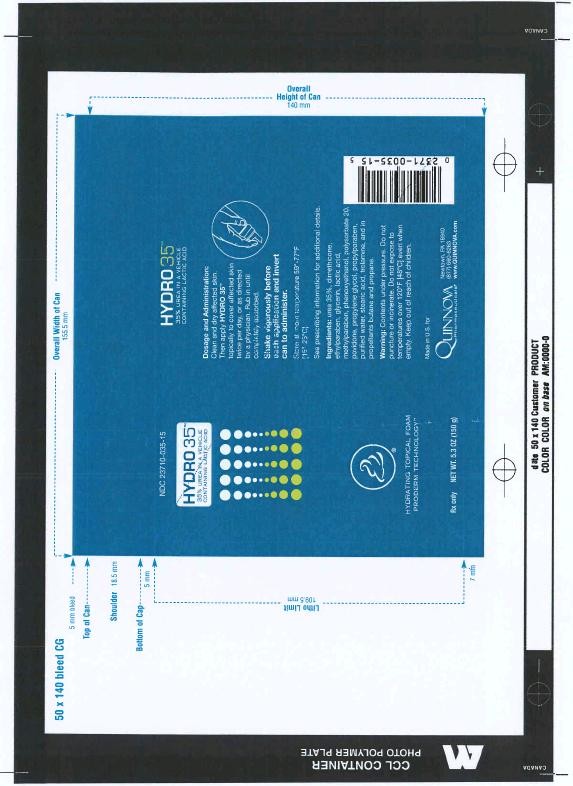
HYDRO 35 is supplied in a 150 gram or 5.3 ounce aerosolized canister bearing the NDC Number 23710-035-15 and a 22 gram or .79 ounce aerosolized canister bearing the NDC Number 23710-035-20. The 22g canister is a physician-dispensed sample product.
Store at controlled room temperature 15° - 25°C (59° - 77°F).
U.S. Patent Pending for HYDRO 35.
HYDRO 35 is manufactured for Quinnova Pharmaceuticals,
Inc., Newtown, PA 18940, (877) 660-6263,
www.QUINNOVA.com.
Prescribing Information as of May 2009.
PRINCIPAL DISPLAY PANEL
~~

~~
DISPLAY PANEL
~~

~~
DISPLAY PANEL
~~
~~
Hydro 35Urea in a water and lipid based foam containing lactic acid AEROSOL, FOAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||