Hydralazine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- HYDRALAZINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- HYDRALAZINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- HYDRALAZINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
HYDRALAZINE HYDROCHLORIDE DESCRIPTION
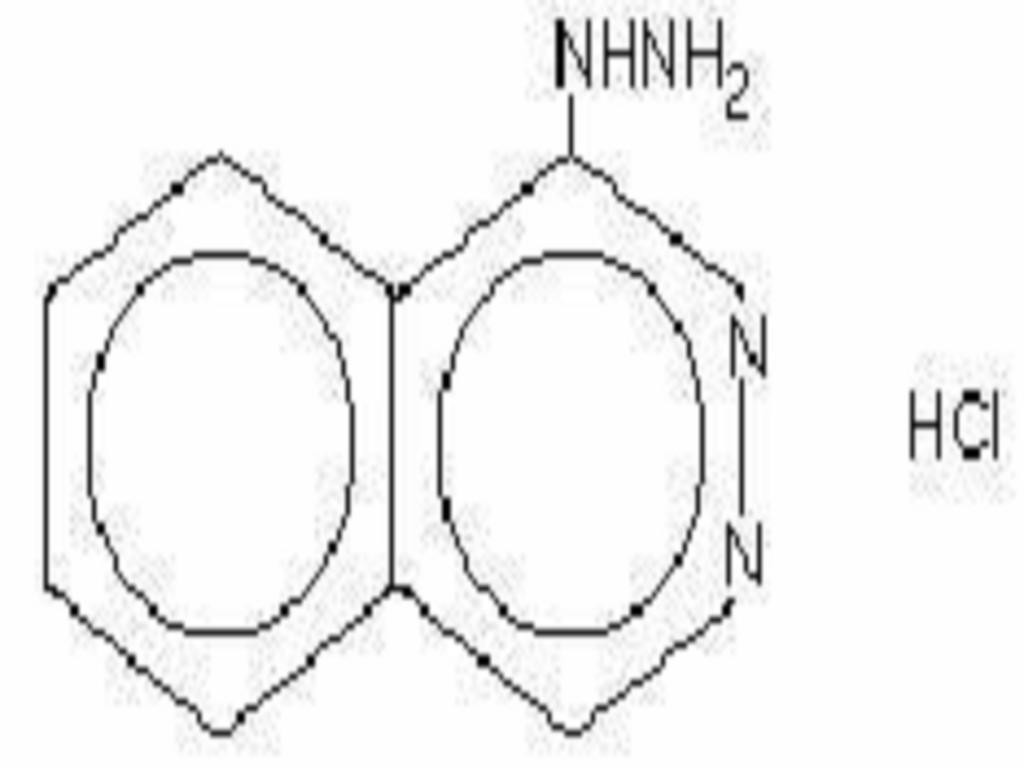
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGYINDICATIONS & USAGE
INDICATIONS AND USAGECONTRAINDICATIONS
CONTRAINDICATIONSWARNINGS
WARNINGSPRECAUTIONS, Laboratory Tests
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
Information for PatientsLABORATORY TESTS
Laboratory TestsDRUG INTERACTIONS
Drug/Drug InteractionsDrug/Food Interactions
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of FertilityPREGNANCY
Pregnancy Category CNURSING MOTHERS
Nursing MothersPEDIATRIC USE
Pediatric UseHYDRALAZINE HYDROCHLORIDE ADVERSE REACTIONS
ADVERSE REACTIONSOVERDOSAGE
OVERDOSAGEDOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATIONHOW SUPPLIED
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:FD&C RED NO. 40
HYPROMELLOSE
ANHYDROUS LACTOSE
MINERAL OIL
CELLULOSE, MICROCRYSTALLINE
MAGNESIUM STEARATE
STARCH, CORN
SODIUM LAURYL SULFATE
TITANIUM DIOXIDE
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Hydralazine HydrochlorideHydralazine Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!