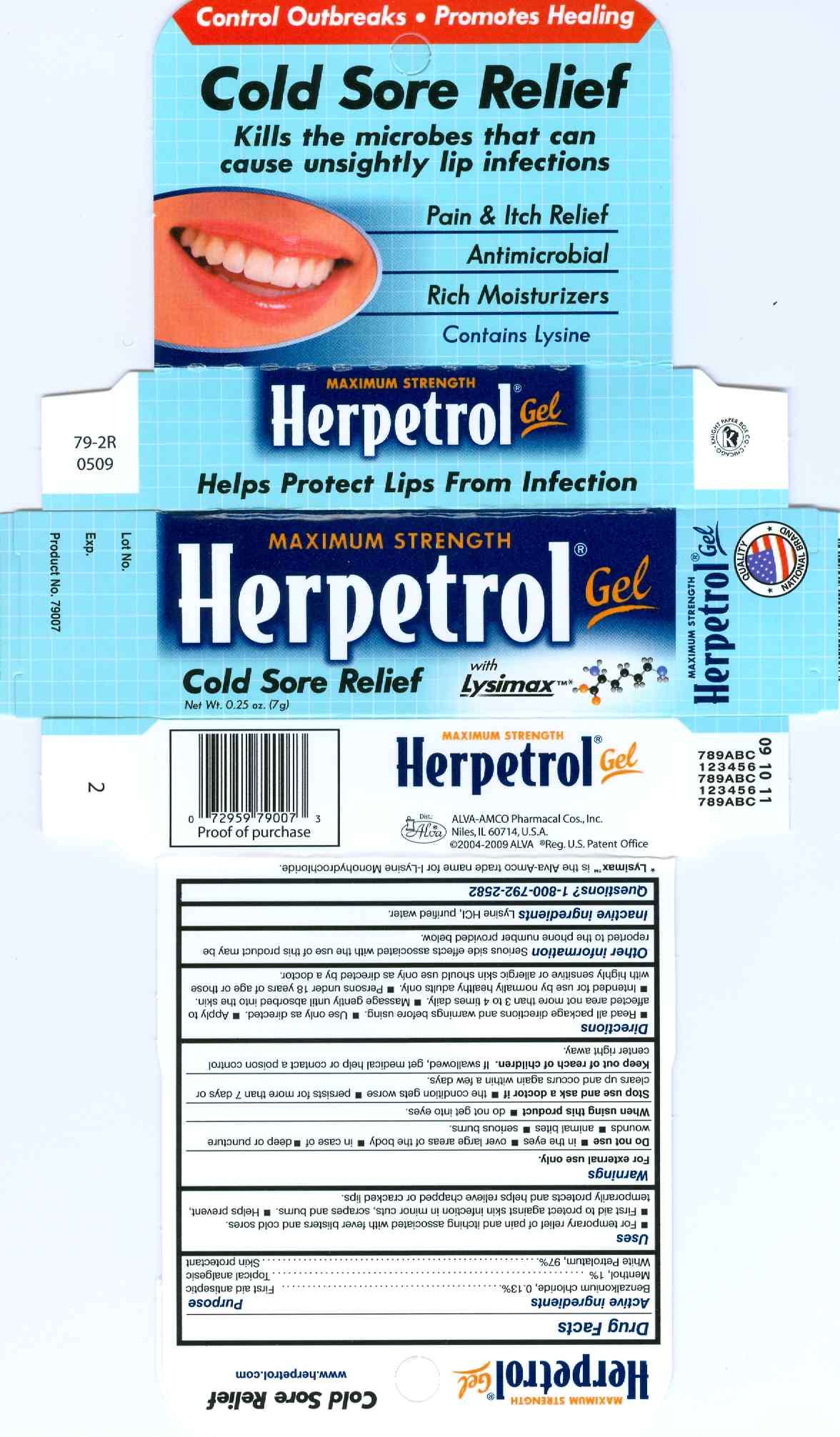
Herpetrol
Alva-Amco Pharmacal Companies, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Herpetrol Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Directions
- Herpetrol Other information
- Inactive ingredients
FULL PRESCRIBING INFORMATION
Active Ingredients
Benzalkonium chloride, 0.13%.............................First aid antiseptic
Menthol, 1%.......................................................Topical analgesic
White petrolatum, 97%........................................Skin protectant
Herpetrol Uses
- For temporary relief of pain and itching associated with fever blisters and cold sores.
- First aid to protect against skin infection in minor cuts, scrapes and burns.
- Helps prevent, temporarily protects and helps relieve chapped or cracked lips.
Warnings
For external use only.
Do not use
- in the eyes
- over large areas of the body
- in case of
-
- deep or puncture wounds
- animal bites
- serious burns
When using this product
- do not get into eyes.
Stop use and ask a doctor if
- the condition gets worse
- persists for more than 7 days or clears up and occurs again within a few days.
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
Directions
- Read all package directions and warnings before using.
- Use only as directed.
- Apply to affected area not more than 3 to 4 times daily.
- Massage gently until absorbed into the skin.
- Intended for use by normally healthy adults only.
- Persons under 18 years of age or those with highly sensitive or allergic skin should use only as directed by a doctor.
Herpetrol Other information
Serious side effects associated with the use of this product may be reported to the phone number provided below.
Inactive ingredients
Lysine HCl, purified water.
Questions? 1-800-792-2582
HerpetrolBenzalkonium chloride, menthol, petrolatum OINTMENT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||