GUNA-ORAL SPRAY
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS/PURPOSE
ACONITUM NAPELLUS 3X ANTI-INFLAMMATORY
ARNICA MONTANA 3X FEVER RELIEF
BELLADONNA 4X SWELLING
ECHINACEA ANGUSTIFOLIA 2X BACTERIAL INFECTION
ECHINACEA PURPUREA 2X IMMUNE REGULATION
HAMAMELIS VIRGINIANA 2X SUPPORTS TISSUE REPAIR
HEPAR SULFURIS CALCAREUM 6X ANTIBACTERIAL IMMUNE SUPPORT
HYPERICUM PERFORATUM 3X ANALGESIC
MILLEFOLIUM 3X ORAL ANTI- INFLAMMATORY
SYMPHYTUM OFFICINALE 6X ORAL HYPERSENSITIVITY
TEETH 12X TOOTH HYPERSENSITIVITY
USES
For the temporary relief of symptoms due to Oro-pharyngeal Inflammatory conditions (Pharyngitis, Gingivitis, or Stomatitis) such as: throat pain, sensitive teeth, mouth pain, hoarseness
WARNINGS
Stop use and ask doctor if symptoms of throat pain and hoarseness worsen or persist more than 3 days
PREGNANCY
If pregnant or breast-feeding ask a doctor before use
WARNINGS
Keep this and all medicines out of reach of children
DIRECTIONS
Adults and children 12 years and older 2 sprays to back of throat, 3-5 times per day
Children between 12 years and 6 years of age 1 spray to back of throat, 3-5 times per day
Children under 6 years 1 spray to back of throat, 3 times per day
QUESTIONS
Questions?: info@gunainc.com
Tel. (484) 223-3500
PRINCIPAL DISPLAY PANEL
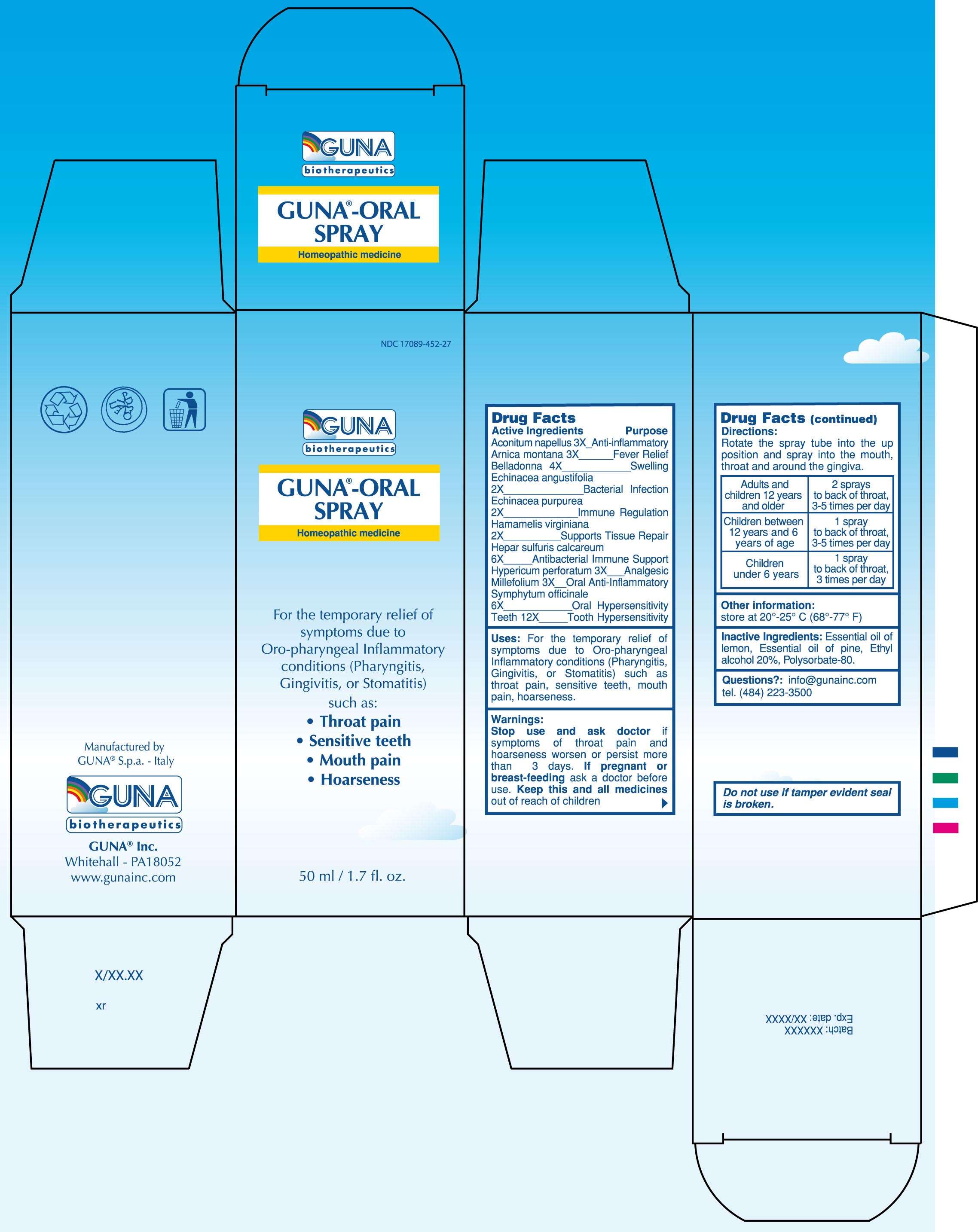
GUNA-ORAL SPRAYACHILLEA MILLEFOLIUM - ACONITUM NAPELLUS - ARNICA MONTANA - ATROPA BELLADONNA - CALCIUM SULFIDE - COMFREY ROOT - ECHINACEA ANGUSTIFOLIA - ECHINACEA PURPUREA - HYPERICUM PERFORATUM - SUS SCROFA TOOTH - WITCH HAZEL - SPRAY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||