GUNA-AWARENESS
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS/PURPOSE
AURUM METALLICUM 12X ANTIDEPRESSANT
BRAIN DERIVED NEUROTROPHIC FACTOR 4C HELPS MENTAL ACTIVITY
BUFO RANA 12X HELPS MENTAL ACTIVITY
CALCAREA CARBONICA 3X IMMUNE STRENGTHENING
CHROMIUM SULPHURICUM 3X DETOXIFICATION
CICUTA VIROSA 12X ANTISPASTIC
COBALAMIN 3X ANTIOXIDANT
COENZYME Q 3X ANTIOXIDANT
CUPRUM METALLICUM 12X ANTISPASTIC
FERRUM METALLICUM 3X ANTIOXIDANT
FOLIC ACID 3X ANTIOXIDANT
FRONTAL LOBE 6X SUPPORTS COORDINATING BEHAVIOR
LACHESIS MUTUS 12X ANTI-INFLAMMATORY
MELATONIN 4C ANTISTRESS
MOLYBDENUM METALLICUM 3X ANTIAGING
NEUROTROPHIN 3 4C SUPPORTS MENTAL ACTIVITY
NEUROTROPHIN 4 4C SUPPORTS MENTAL ACTIVITY
OXYTOCIN 6X ANTISPASTIC
SILICEA 12X IMMUNE STRENGTHENING
TEMPORAL LOBE 6X SUPPORTS AUDITORY PROCESSING
THYROTROPIN-RELEASING HORMONE 3X HELPS MENTAL ACTIVITY
TRIMETHYLGLYCINE 3X DETOXIFICATION
VANADIUM METALLICUM 6X ANTIOXIDANT
ZINCUM METALLICUM 3X ANTISTRESS
USES
For the temporary relief of symptoms of Impaired Cognitive Function such as: Inability to Concentrate, Restlessness, Easy distractibility
WARNINGS
Stop use and ask doctor if symptoms of Inability to Concentrate, Restlessness, Easy distractibility worsen or persist more than 5 days
PREGNANCY
If pregnant or breast-feeding ask a doctor before use
WARNINGS
Keep this and all medicines out of reach of children
DIRECTIONS
Take 15 minutes before meals
Adults and children 12 years and older 20 drops in a little water, 2 times per day
Children between 12 years and 6 years of age 10 drops in a little water, 2 times per day
Children under 6 years 5 drops in a glass of water, 2 times per day
QUESTIONS
Questions?: info@gunainc.com, tel. (484) 223-3500
PRINCIPAL DISPLAY PANEL
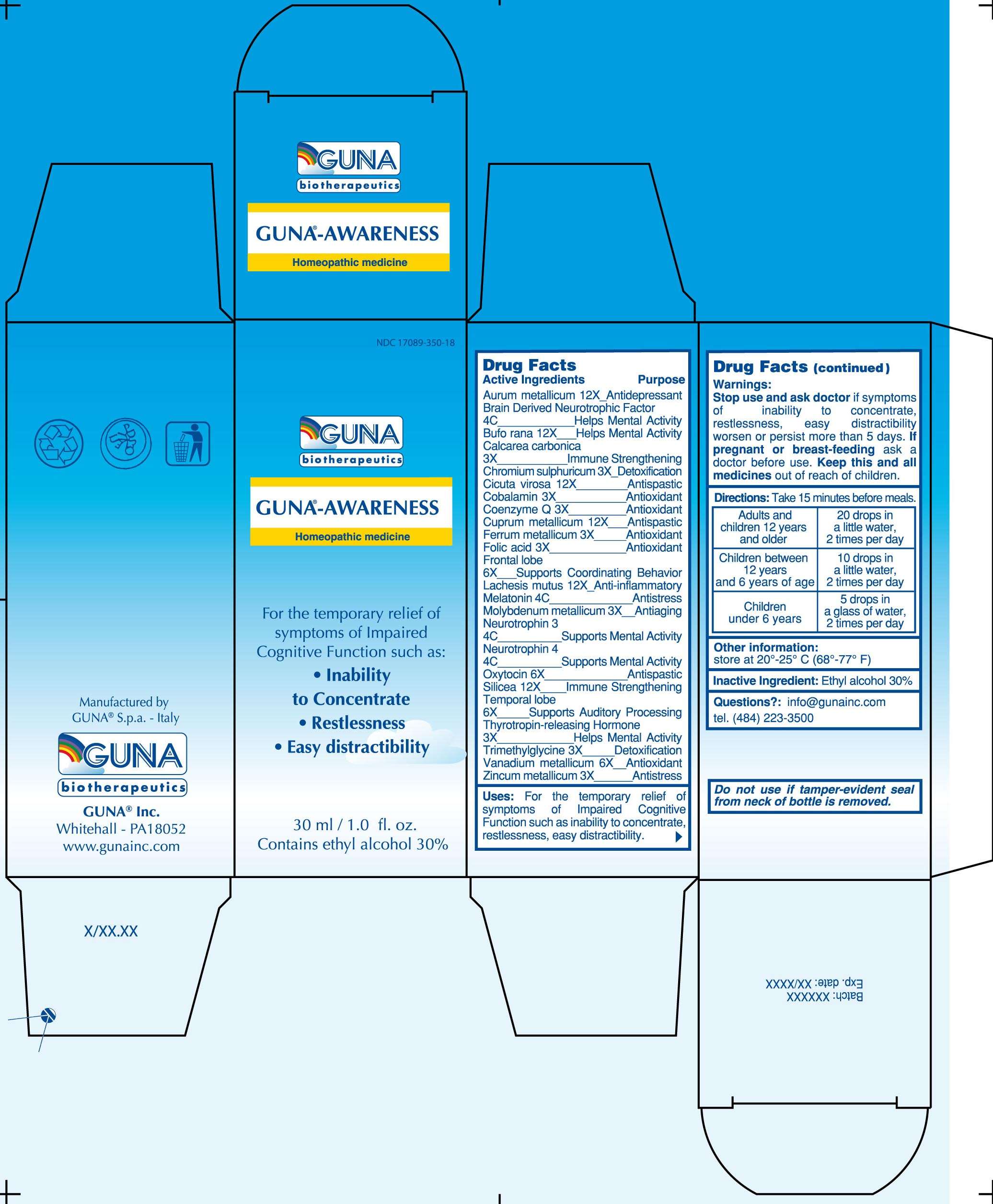
GUNA-AWARENESSBETAINE - BUFO BUFO CUTANEOUS GLAND - CALCIUM CARBONATE - CHROMIC SULFATE - CICUTA VIROSA ROOT - COPPER - FOLIC ACID - GOLD - COBALAMIN - IRON - LACHESIS MUTA VENOM - MELATONIN - MOLYBDENUM - NEUROTROPHIN-3 - NEUROTROPHIN-4 - OXYTOCIN - SILICON DIOXIDE - SUS SCROFA FRONTAL LOBE - SUS SCROFA TEMPORAL LOBE - THYROTROPIN ALFA - UBIDECARENONE - VANADIUM - ZINC - BRAIN-DERIVED NEUROTROPHIC FACTOR HUMAN - SOLUTION/ DROPS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||