good sense nighttime sleep aid
Perrigo Nighttime Sleep Aid Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
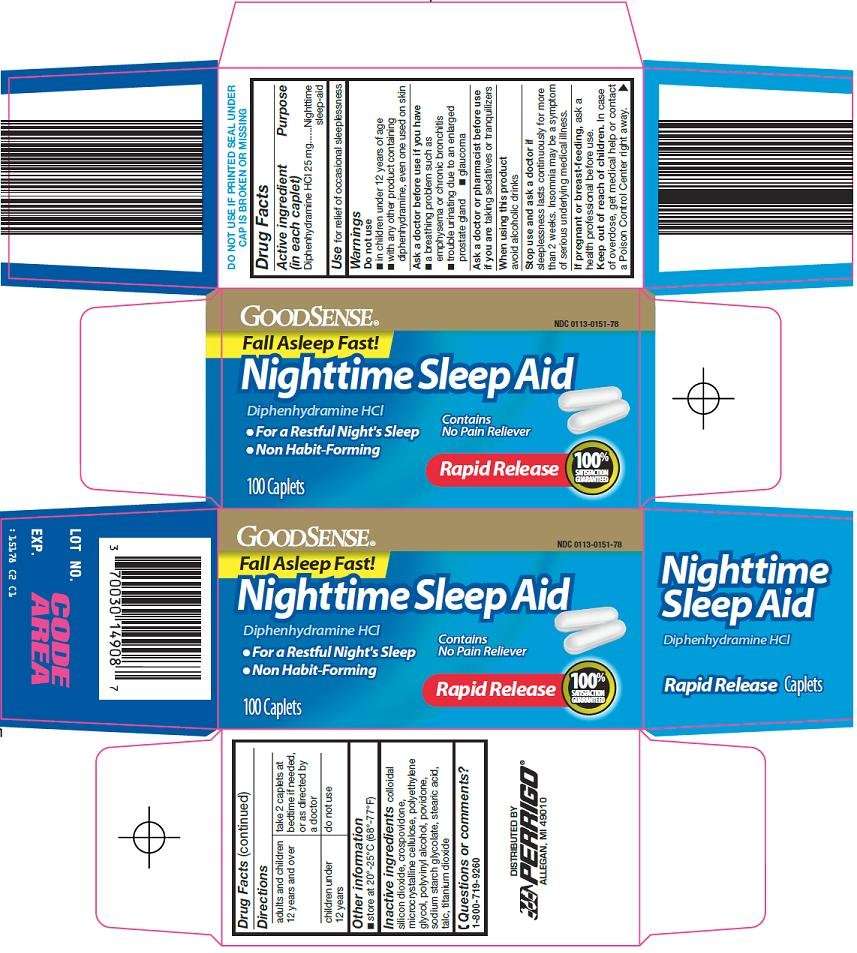
- Active ingredient (in each caplet)
- Purpose
- good sense nighttime sleep aid Uses
- Warnings
- Directions
- good sense nighttime sleep aid Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient (in each caplet)
Diphenhydramine HCl 25 mg
Purpose
Nighttime sleep-aid
good sense nighttime sleep aid Uses
for relief of occasional sleeplessness
Warnings
Do not use
- in children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers
When using this product
avoid alcoholic drinks
Stop use and ask a doctor if
sleeplessness lasts continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 12 years and over | take 2 caplets at bedtime if needed, or as directed by a doctor |
| children under 12 years | do not use |
good sense nighttime sleep aid Other information
- store at 20º-25ºC (68º-77ºF)
Inactive ingredients
colloidal silicon dioxide, crospovidone, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, sodium starch glycolate, stearic acid, talc, titanium dioxide
Questions or comments?
1-800-719-9260
Principal Display Panel
Fall Asleep Fast!
Nighttime Sleep Aid
Diphenhydramine HCl
Contains no Pain Reliever
For a Restful Night’s Sleep
Non habit-forming
Rapid Release
Nighttime Sleep Aid Carton
good sense nighttime sleep aidDiphenhydramine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!