GLIPIZIDE
FULL PRESCRIBING INFORMATION: CONTENTS*
- GLIPIZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- GLIPIZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- GLIPIZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
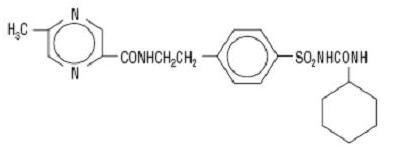
GLIPIZIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionPharmacokineticsbelow).
Other Effects
Pharmacokinetics
INDICATIONS & USAGE
GLIPIZIDE CONTRAINDICATIONS
WARNINGS
SPECIAL WARNING ON INCREASED RISK OF CARDIOVASCULAR MORTALITYThe administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups (Diabetes, 19, supp. 2:747-830, 1970).
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 21/2 times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of glipizide and of alternative modes of therapy.
Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
PRECAUTIONS
GeneralInformation for Patients
Physician Counseling Information for Patients
Drug Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy
Nursing Mothers
Pediatric Use
Geriatric Use
GLIPIZIDE ADVERSE REACTIONS
Hypoglycemia
PRECAUTIONSandOVERDOSAGEsections.
Gastrointestinal
Gastrointestinal disturbances are the most common reactions. Gastrointestinal complaints were reported with the following apprroximate incidence: nuasea and diarrhea, one in seventy; constipation and gastralgia, one in one hundred. They appear to be dose-related and may disappear on division or reduction of dosage. Cholestatic jaundice may occur rarely with sulfonyluras: Glipizide should be discontinued if this occurs.
Dermatologic
Allergic skin reactions including erythema, morbilliform of maculopapular eruptions, urticaria, pruritus, and eczema have been reported in about one in seventy patients. These may be transent and may disappear despite continued use of glipizide; if skin reactions persist, the drug should be discontinued. Porphyria cutanea tarda and photosensitivity reactions have been reported with sulfonyluras.
Hematologic
Metabolic
Endocrine Reactions
Miscellaneous
Laboratory Tests
Postmarketing Experience
The following adverse events have been reported in postmarketing surveillance:
OVERDOSAGE
DOSAGE & ADMINISTRATION
Initial Dose
Titration
Maintenance
Patients Receiving Insulin
Patients Receiving Other Oral Hypoglycemic Agents
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

GLIPIZIDEGLIPIZIDE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!