Glipizide
FULL PRESCRIBING INFORMATION: CONTENTS*
- GLIPIZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- GLIPIZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- GLIPIZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
GLIPIZIDE DESCRIPTION
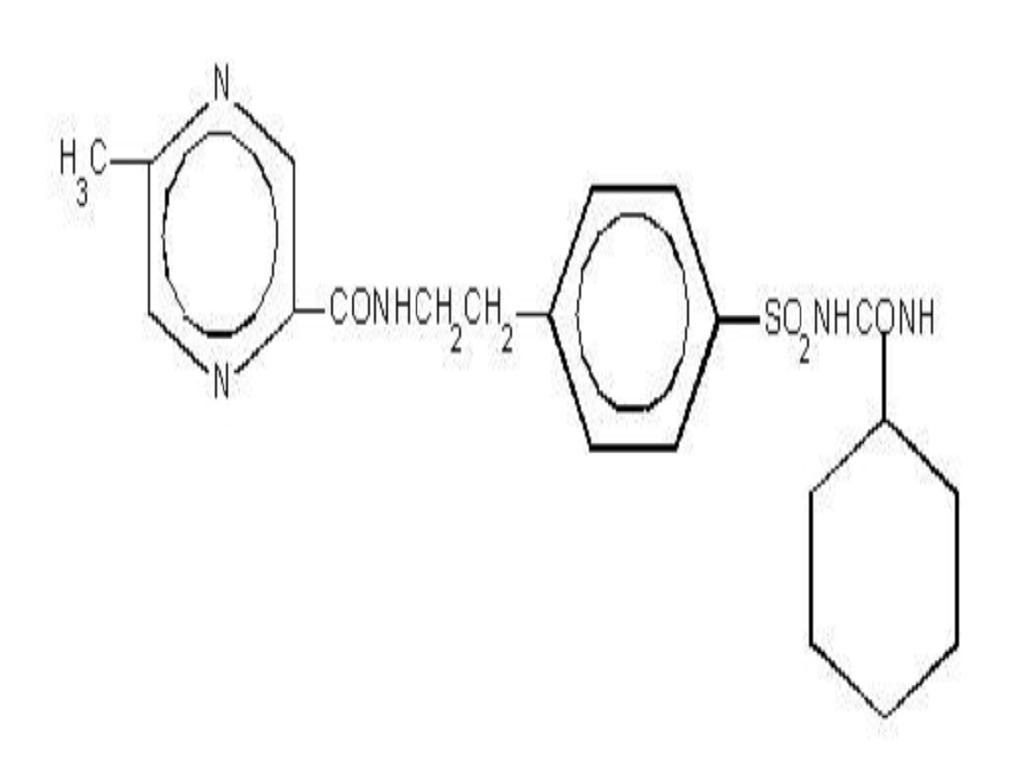
System Components and Performance
CLINICAL PHARMACOLOGY
Mechanism of Action
Effects on Blood Glucose
Other Effects
Pharmacokinetics and Metabolism
INDICATIONS & USAGE
GLIPIZIDE CONTRAINDICATIONS
WARNINGS
SPECIAL WARNING ON INCREASED RISK OF CARDIOVASCULAR MORTALITYThe administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with type 2 diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups (Diabetes, 19, SUPP. 2: 747830, 1970).
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of glipizide and of alternative modes of therapy.
Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
PRECAUTIONS
GeneralRenal and Hepatic Disease
GI Disease
Hypoglycemia
Loss of Control of Blood Glucose
Laboratory Tests
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Category CNonteratogenic Effects
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATIONGLIPIZIDE ADVERSE REACTIONS
Hypoglycemia:PRECAUTIONSOVERDOSAGE
Glipizide Extended-Release Tablets (%) (N=278)Placebo (%) (N=69)Adverse Effect
Hematologic:
Metabolic:
Endocrine Reactions:
Laboratory Tests:
OVERDOSAGE
DOSAGE & ADMINISTRATION
Recommended Dosing
PRECAUTIONS
Combination Use
Patients Receiving Insulin
Patients Receiving Other Oral Hypoglycemic Agents
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
GLIPIZIDE ER(glipizide extended release tablets)
What is GLIPIZIDE ER?
-
● your body may not be making enough insulin
-
● your body may not be using the insulin that you have already made
-
● the level of sugar in your blood is too high
-
● helping the body release more of its own insulin
-
● helping the body respond better to its own insulin
-
● lowering the amount of sugar (glucose) made by the body
Do not use GLIPIZIDE ER if you:
-
● have a condition called diabetic ketoacidosis
-
● have ever had an allergic reaction to glipizide or any of the other ingredients in GLIPIZIDE ER. Ask your healthcare provider or pharmacist for a list of these ingredients.
-
● are taking or using any prescription medicines or non-prescription medicines, including natural or herbal remedies. Other medications can increase your chance of getting low blood sugar or high blood sugar. Be sure to tell your healthcare provider if you take the medicines miconazole or fluconazole, used to fight fungus infections.
-
● have ever had a condition called diabetic ketoacidosis
-
● have kidney or liver problems
-
● have had blockage or narrowing of your intestines due to illness or past surgery
-
● have chronic (continuing) diarrhea
-
● are pregnant or might be pregnant. Your healthcare provider may switch you to insulin injections some time during your pregnancy. You should not take GLIPIZIDE ER during the last month of pregnancy
-
● are breast-feeding. GLIPIZIDE ER may pass to the baby through your milk and cause harm.
-
● Take GLIPIZIDE ER once a day with breakfast. The tablet is designed to release the medicine slowly over 24 hours. This is why you have to take it only once a day.
-
● Swallow the tablet whole. Never chew, crush or cut the tablet in half. This would damage the tablet and release too much medicine into your body at one time.
-
● After all of the medicine has been released, the empty tablet shell will pass out of the body normally in a bowel movement. Do not be concerned if you see the empty tablet shell in your stool (bowel movement).
What Should I Avoid While Taking GLIPIZIDE ER?
What are the Possible Side Effects of GLIPIZIDE ER?
Low blood sugar.
-
● a cold clammy feeling
-
● unusual sweating
-
● dizziness
-
● weakness
-
● trembling
-
● shakiness
-
● hunger
-
● fast heartbeat
-
● headache
-
● blurred vision
-
● slurred speech
-
● tingling in the lips or hands
Other side effects
-
● feeling jittery
-
● diarrhea
-
● gas
How To Store GLIPIZIDE ER
General Advice About Prescription Medicines
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

GlipizideGlipizide TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!