Glipizide
FULL PRESCRIBING INFORMATION: CONTENTS*
- GLIPIZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- GLIPIZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LABORATORY TESTS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- GLIPIZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
GLIPIZIDE DESCRIPTION
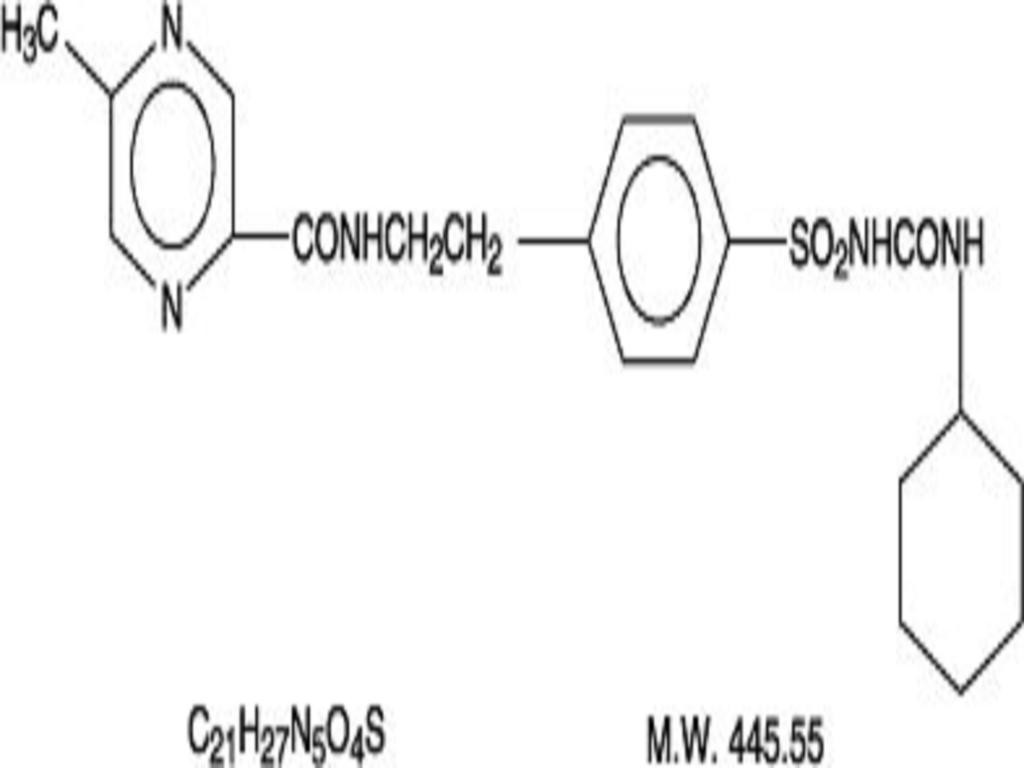
CLINICAL PHARMACOLOGY
Mechanism of ActionPharmacokinetics
Other Effects
PHARMACOKINETICS
INDICATIONS & USAGE
GLIPIZIDE CONTRAINDICATIONS
WARNINGS
SPECIAL WARNING ON INCREASED RISK OF CARDIOVASCULAR MORTALITYPRECAUTIONS
GeneralHemolytic Anemia
Macrovascular Outcomes
Renal and Hepatic Disease
Hypoglycemia
Loss of Control of Blood Glucose
LABORATORY TESTS
INFORMATION FOR PATIENTS
Physician Counseling Information for Patients
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Category CNonteratogenic Effects
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
GLIPIZIDE ADVERSE REACTIONS
Hypoglycemia
PRECAUTIONSOVERDOSAGE
Gastrointestinal
Dermatologic
Hematologic
Metabolic
Endocrine Reactions
Miscellaneous
Laboratory Tests
OVERDOSAGE
DOSAGE & ADMINISTRATION
Initial Dose
Titration
Maintenance
PRECAUTIONS
Patients Receiving Insulin
Patients Receiving Other Oral Hypoglycemic Agents
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

GlipizideGlipizide TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!