Glimepiride
Glimepiride Tablets, USP 1 mg, 2 mg and 4 mg
FULL PRESCRIBING INFORMATION: CONTENTS*
- GLIMEPIRIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- GLIMEPIRIDE INDICATIONS AND USAGE
- GLIMEPIRIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- GLIMEPIRIDE ADVERSE REACTIONS
- OVERDOSAGE
- GLIMEPIRIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- ANIMAL TOXICOLOGY
- HUMAN OPHTHALMOLOGY DATA
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Rx Only
GLIMEPIRIDE DESCRIPTION
Glimepiride Tablets, USP are an oral blood-glucose-lowering drug of the sulfonylurea class. Glimepiride, USP is a white to yellowish-white, crystalline, odorless to practically odorless powder formulated into tablets of 1-mg, 2-mg, and 4-mg strengths for oral administration. Glimepiride Tablets, USP contain the active ingredient glimepiride, USP and the following inactive ingredients: lactose monohydrate, magnesium stearate, povidone, and sodium starch glycolate. In addition, Glimepiride 1-mg tablets contain Ferric Oxide (Iron Oxide Red), Glimepiride 2-mg tablets contain Ferric Oxide (Iron Oxide Yellow), and FD&C Blue #2 Aluminum Lake, and Glimepiride 4-mg tablets contain FD&C Blue #2 Aluminum Lake.
Chemically, glimepiride is identified as 1-[[p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl]phenyl]sulfonyl]-3-(trans-4-methylcyclohexyl)urea.
The CAS Registry Number is 93479-97-1
The structural formula is:
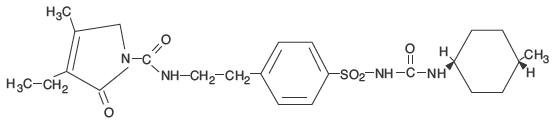
Molecular Formula: C24H34N4O5S
Molecular Weight: 490.62
Glimepiride is practically insoluble in water.
CLINICAL PHARMACOLOGY
The primary mechanism of action of glimepiride in lowering blood glucose appears to be dependent on stimulating the release of insulin from functioning pancreatic beta cells. In addition, extrapancreatic effects may also play a role in the activity of sulfonylureas such as glimepiride. This is supported by both preclinical and clinical studies demonstrating that glimepiride administration can lead to increased sensitivity of peripheral tissues to insulin. These findings are consistent with the results of a long-term, randomized, placebo-controlled trial in which glimepiride therapy improved postprandial insulin/C-peptide responses and overall glycemic control without producing clinically meaningful increases in fasting insulin/C-peptide levels. However, as with other sulfonylureas, the mechanism by which glimepiride lowers blood glucose during long-term administration has not been clearly established.
Glimepiride is effective as initial drug therapy. In patients where monotherapy with glimepiride or metformin has not produced adequate glycemic control, the combination of glimepiride and metformin may have a synergistic effect, since both agents act to improve glucose tolerance by different primary mechanisms of action. This complementary effect has been observed with metformin and other sulfonylureas, in multiple studies.
A mild glucose-lowering effect first appeared following single oral doses as low as 0.5-0.6 mg in healthy subjects. The time required to reach the maximum effect (i.e., minimum blood glucose level [Tmin]) was about 2 to 3 hours. In noninsulin-dependent (Type 2) diabetes mellitus (NIDDM) patients, both fasting and 2-hour postprandial glucose levels were significantly lower with glimepiride (1, 2, 4, and 8 mg once daily) than with placebo after 14 days of oral dosing. The glucose-lowering effect in all active treatment groups was maintained over 24 hours.
In larger dose-ranging studies, blood glucose and HbA1c were found to respond in a dose-dependent manner over the range of 1 to 4 mg/day of glimepiride. Some patients, particularly those with higher fasting plasma glucose (FPG) levels, may benefit from doses of glimepiride up to 8 mg once daily. No difference in response was found when glimepiride was administered once or twice daily.
In two 14-week, placebo-controlled studies in 720 subjects, the average net reduction in HbA1c for glimepiride tablets patients treated with 8 mg once daily was 2.0% in absolute units compared with placebo-treated patients. In a long-term, randomized, placebo-controlled study of Type 2 diabetic patients unresponsive to dietary management, glimepiride therapy improved postprandial insulin/C-peptide responses, and 75% of patients achieved and maintained control of blood glucose and HbA1c. Efficacy results were not affected by age, gender, weight, or race.
In long-term extension trials with previously-treated patients, no meaningful deterioration in mean fasting blood glucose (FBG) or HbA1c levels was seen after 2 1/2 years of glimepiride therapy.
Combination therapy with glimepiride and insulin (70% NPH/30% regular) was compared to placebo/insulin in secondary failure patients whose body weight was >130% of their ideal body weight. Initially, 5-10 units of insulin were administered with the main evening meal and titrated upward weekly to achieve predefined FPG values. Both groups in this double blind study achieved similar reductions in FPG levels but the glimepiride/insulin therapy group used approximately 38% less insulin.
Glimepiride therapy is effective in controlling blood glucose without deleterious changes in the plasma lipoprotein profiles of patients treated for Type 2 diabetes.
After oral administration, glimepiride is completely (100%) absorbed from the GI tract. Studies with single oral doses in normal subjects and with multiple oral doses in patients with Type 2 diabetes have shown significant absorption of glimepiride within 1 hour after administration and peak drug levels (Cmax) at 2 to 3 hours. When glimepiride was given with meals, the mean Tmax (time to reach Cmax) was slightly increased (12%) and the mean Cmax and AUC (area under the curve) were slightly decreased (8% and 9%, respectively).
After intravenous (IV) dosing in normal subjects, the volume of distribution (Vd) was 8.8 L (113 mL/kg), and the total body clearance (CL) was 47.8 mL/min. Protein binding was greater than 99.5%.
Glimepiride is completely metabolized by oxidative biotransformation after either an IV or oral dose. The major metabolites are the cyclohexyl hydroxy methyl derivative (M1) and the carboxyl derivative (M2). Cytochrome P450 2C9 has been shown to be involved in the biotransformation of glimepiride to M1. M1 is further metabolized to M2 by one or several cytosolic enzymes. M1, but not M2, possesses about 1/3 of the pharmacological activity as compared to its parent in an animal model; however, whether the glucose-lowering effect of M1 is clinically meaningful is not clear.
When14C-glimepiride was given orally, approximately 60% of the total radioactivity was recovered in the urine in 7 days and M1 (predominant) and M2 accounted for 80-90% of that recovered in the urine. Approximately 40% of the total radioactivity was recovered in feces and M1 and M2 (predominant) accounted for about 70% of that recovered in feces. No parent drug was recovered from urine or feces. After IV dosing in patients, no significant biliary excretion of glimepiride or its M1 metabolite has been observed.
The pharmacokinetic parameters of glimepiride obtained from a single-dose, crossover, dose-proportionality (1, 2, 4, and 8 mg) study in normal subjects and from a single- and multiple-dose, parallel, dose-proportionality (4 and 8 mg) study in patients with Type 2 diabetes are summarized below:
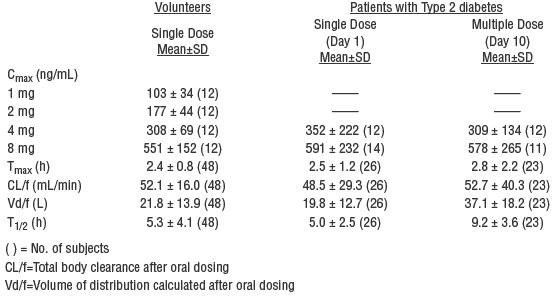
These data indicate that glimepiride did not accumulate in serum, and the pharmacokinetics of glimepiride were not different in healthy volunteers and in Type 2 diabetic patients. Oral clearance of glimepiride did not change over the 1-8-mg dose range, indicating linear pharmacokinetics.
In normal healthy volunteers, the intra-individual variabilities of Cmax, AUC, and CL/f for glimepiride were 23%, 17%, and 15%, respectively, and the inter-individual variabilities were 25%, 29%, and 24%, respectively.
Comparison of glimepiride pharmacokinetics in Type 2 diabetic patients ≤65 years and those >65 years was performed in a study using a dosing regimen of 6 mg daily. There were no significant differences in glimepiride pharmacokinetics between the two age groups. The mean AUC at steady state for the older patients was about 13% lower than that for the younger patients; the mean weight-adjusted clearance for the older patients was about 11% higher than that for the younger patients.
The pharmacokinetics of glimepiride (1 mg) were evaluated in a single dose study conducted in 30 Type 2 diabetic patients (Male = 7; Female = 23) between ages 10 and 17 years. The mean AUC(0-last) (338.8±203.1 ng•hr/mL), Cmax (102.4±47.7 ng/mL) and T1/2(3.1±1.7 hours) were comparable to those previously reported in adults (AUC(0-last) 315.2±95.9 ng•hr/mL, Cmax 103.2±34.3 ng/mL and T1/2 5.3±4.1 hours).
There were no differences between males and females in the pharmacokinetics of glimepiride when adjustment was made for differences in body weight.
No pharmacokinetic studies to assess the effects of race have been performed, but in placebo-controlled studies of glimepiride tablets in patients with Type 2 diabetes, the antihyperglycemic effect was comparable in whites (n = 536), blacks (n = 63), and Hispanics (n = 63).
A single-dose, open-label study was conducted in 15 patients with renal impairment. Glimepiride (3 mg) was administered to 3 groups of patients with different levels of mean creatinine clearance (CLcr); (Group I, CLcr = 77.7 mL/min, n = 5), (Group II, CLcr = 27.7 mL/min, n = 3), and (Group III, CLcr = 9.4 mL/min, n = 7). Glimepiride was found to be well tolerated in all 3 groups. The results showed that glimepiride serum levels decreased as renal function decreased. However, M1 and M2 serum levels (mean AUC values) increased 2.3 and 8.6 times from Group I to Group III. The apparent terminal half-life (T1/2) for glimepiride did not change, while the half-lives for M1 and M2 increased as renal function decreased. Mean urinary excretion of M1 plus M2 as percent of dose, however, decreased (44.4%, 21.9%, and 9.3% for Groups I to III).
A multiple-dose titration study was also conducted in 16 Type 2 diabetic patients with renal impairment using doses ranging from 1-8 mg daily for 3 months. The results were consistent with those observed after single doses. All patients with a CLcr less than 22 mL/min had adequate control of their glucose levels with a dosage regimen of only 1 mg daily. The results from this study suggested that a starting dose of 1 mg glimepiride may be given to Type 2 diabetic patients with kidney disease, and the dose may be titrated based on fasting blood glucose levels.
No studies were performed in patients with hepatic insufficiency.
There were no important differences in glimepiride metabolism in subjects identified as phenotypically different drug-metabolizers by their metabolism of sparteine.
The pharmacokinetics of glimepiride in morbidly obese patients were similar to those in the normal weight group, except for a lower Cmax and AUC. However, since neither Cmax nor AUC values were normalized for body surface area, the lower values of Cmax and AUC for the obese patients were likely the result of their excess weight and not due to a difference in the kinetics of glimepiride.
The hypoglycemic action of sulfonylureas may be potentiated by certain drugs, including nonsteroidal anti-inflammatory drugs, clarithromycin, disopyramide, fluoxetine, and quinolones and other drugs that are highly protein bound, such as salicylates, sulfonamides, chloramphenicol, coumarins, probenecid, monoamine oxidase inhibitors, and beta adrenergic blocking agents. When these drugs are administered to a patient receiving glimepiride, the patient should be observed closely for hypoglycemia. When these drugs are withdrawn from a patient receiving glimepiride, the patient should be observed closely for loss of glycemic control.
A potential interaction between oral miconazole and oral hypoglycemic agents leading to severe hypoglycemia has been reported. Whether this interaction also occurs with the intravenous, topical, or vaginal preparations of miconazole is not known.
Certain drugs tend to produce hyperglycemia and may lead to loss of control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, and isoniazid. When these drugs are administered to a patient receiving glimepiride, the patient should be closely observed for loss of control. When these drugs are withdrawn from a patient receiving glimepiride, the patient should be observed closely for hypoglycemia.
Coadministration of aspirin (1 g tid) and glimepiride led to a 34% decrease in the mean glimepiride AUC and, therefore, a 34% increase in the mean CL/f. The mean Cmax had a decrease of 4%. Blood glucose and serum C-peptide concentrations were unaffected and no hypoglycemic symptoms were reported. Pooled data from clinical trials showed no evidence of clinically significant adverse interactions with uncontrolled concurrent administration of aspirin and other salicylates.
Coadministration of either cimetidine (800 mg once daily) or ranitidine (150 mg bid) with a single 4-mg oral dose of glimepiride did not significantly alter the absorption and disposition of glimepiride, and no differences were seen in hypoglycemic symptomatology. Pooled data from clinical trials showed no evidence of clinically significant adverse interactions with uncontrolled concurrent administration of H2-receptor antagonists.
Concomitant administration of propranolol (40 mg tid) and glimepiride significantly increased Cmax, AUC, and T1/2 of glimepiride by 23%, 22%, and 15%, respectively, and it decreased CL/f by 18%. The recovery of M1 and M2 from urine, however, did not change. The pharmacodynamic responses to glimepiride were nearly identical in normal subjects receiving propranolol and placebo. Pooled data from clinical trials in patients with Type 2 diabetes showed no evidence of clinically significant adverse interactions with uncontrolled concurrent administration of beta-blockers. However, if beta-blockers are used, caution should be exercised and patients should be warned about the potential for hypoglycemia.
Concomitant administration of glimepiride tablets (4 mg once daily) did not alter the pharmacokinetic characteristics of R- and S-warfarin enantiomers following administration of a single dose (25 mg) of racemic warfarin to healthy subjects. No changes were observed in warfarin plasma protein binding. Glimepiride treatment did result in a slight, but statistically significant, decrease in the pharmacodynamic response to warfarin. The reductions in mean area under the prothrombin time (PT) curve and maximum PT values during glimepiride treatment were very small (3.3% and 9.9%, respectively) and are unlikely to be clinically important.
The responses of serum glucose, insulin, C-peptide, and plasma glucagon to 2 mg glimepiride were unaffected by coadministration of ramipril (an ACE inhibitor) 5 mg once daily in normal subjects. No hypoglycemic symptoms were reported. Pooled data from clinical trials in patients with Type 2 diabetes showed no evidence of clinically significant adverse interactions with uncontrolled concurrent administration of ACE inhibitors.
There is a potential interaction of glimepiride with inhibitors (e.g., fluconazole) and inducers (e.g., rifampicin) of cytochrome P450 2C9.
Although no specific interaction studies were performed, pooled data from clinical trials showed no evidence of clinically significant adverse interactions with uncontrolled concurrent administration of calcium-channel blockers, estrogens, fibrates, NSAIDS, HMG CoA reductase inhibitors, sulfonamides, or thyroid hormone.
GLIMEPIRIDE INDICATIONS AND USAGE
Glimepiride Tablets, USP are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (See DOSAGE AND ADMINISTRATION section).
GLIMEPIRIDE CONTRAINDICATIONS
Glimepiride Tablets, USP are contraindicated in patients with
1. Known hypersensitivity to the drug.
2. Diabetic ketoacidosis, with or without coma. This condition should be treated with insulin.
WARNINGS
WARNINGS
SPECIAL WARNING ON INCREASED RISK OF CARDIOVASCULAR MORTALITY
The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term, prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups (Diabetes, 19 supp. 2: 747-830, 1970).
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2-1/2 times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of glimepiride tablets and of alternative modes of therapy.
Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
Persons allergic to other sulfonamide derivatives may develop an allergic reaction to glimepiride as well.
PRECAUTIONS
General
Macrovascular Outcomes: There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with glimepiride or any other anti-diabetic drug.
Hypoglycemia: All sulfonylurea drugs are capable of producing severe hypoglycemia. Proper patient selection, dosage, and instructions are important to avoid hypoglycemic episodes. Patients with impaired renal function may be more sensitive to the glucose-lowering effect of glimepiride. A starting dose of 1 mg once daily followed by appropriate dose titration is recommended in those patients. Debilitated or malnourished patients, and those with adrenal, pituitary, or hepatic insufficiency are particularly susceptible to the hypoglycemic action of glucoselowering drugs. Hypoglycemia may be difficult to recognize in patients with autonomic neuropathy, the elderly and in people who are taking beta-adrenergic blocking drugs or other sympatholytic agents. Hypoglycemia is more likely to occur when caloric intake is deficient, after severe or prolonged exercise, when alcohol is ingested, or when more than one glucose-lowering drug is used. Combined use of glimepiride with insulin or metformin may increase the potential for hypoglycemia.
Loss of control of blood glucose: When a patient stabilized on any diabetic regimen is exposed to stress such as fever, trauma, infection, or surgery, a loss of control may occur. At such times, it may be necessary to add insulin in combination with glimepiride or even use insulin monotherapy. The effectiveness of any oral hypoglycemic drug, including glimepiride, in lowering blood glucose to a desired level decreases in many patients over a period of time, which may be due to progression of the severity of the diabetes or to diminished responsiveness to the drug. This phenomenon is known as secondary failure, to distinguish it from primary failure in which the drug is ineffective in an individual patient when first given. Should secondary failure occur with glimepiride or metformin monotherapy, combined therapy with glimepiride and metformin or glimepiride and insulin may result in a response. Should secondary failure occur with combined glimepiride/metformin therapy, it may be necessary to initiate insulin therapy.
Hemolytic anemia: Treatment of patients with glucose 6-phosphate dehydrogenase (G6DP) deficiency with sulfonylurea agents can lead to hemolytic anemia. Because glimepiride belongs to the class of sulfonylurea agents, caution should be used in patients with G6PD deficiency and a non-sulfonylurea alternative should be considered. In postmarketing reports, hemolytic anemia has also been reported in patients who did not have known G6PD deficiency.
Patients should be informed of the potential risks and advantages of glimepiride and of alternative modes of therapy. They should also be informed about the importance of adherence to dietary instructions, of a regular exercise program, and of regular testing of blood glucose.
The risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and responsible family members. The potential for primary and secondary failure should also be explained.
Fasting blood glucose should be monitored periodically to determine therapeutic response. Glycosylated hemoglobin should also be monitored, usually every 3 to 6 months, to more precisely assess long-term glycemic control.
(See CLINICAL PHARMACOLOGY, Drug Interactions.)
Studies in rats at doses of up to 5000 ppm in complete feed (approximately 340 times the maximum recommended human dose, based on surface area) for 30 months showed no evidence of carcinogenesis. In mice, administration of glimepiride for 24 months resulted in an increase in benign pancreatic adenoma formation which was dose related and is thought to be the result of chronic pancreatic stimulation. The no-effect dose for adenoma formation in mice in this study was 320 ppm in complete feed, or 46-54 mg/kg body weight/day. This is about 35 times the maximum human recommended dose of 8 mg once daily based on surface area.
Glimepiride was non-mutagenic in a battery of in vitro and in vivo mutagenicity studies (Ames test, somatic cell mutation, chromosomal aberration, unscheduled DNA synthesis, mouse micronucleus test).
There was no effect of glimepiride on male mouse fertility in animals exposed up to 2500 mg/kg body weight (>1,700 times the maximum recommended human dose based on surface area). Glimepiride had no effect on the fertility of male and female rats administered up to 4000 mg/kg body weight (approximately 4,000 times the maximum recommended human dose based on surface area).
Pregnancy Category C
Glimepiride did not produce teratogenic effects in rats exposed orally up to 4000 mg/ kg body weight (approximately 4,000 times the maximum recommended human dose based on surface area) or in rabbits exposed up to 32 mg/kg body weight (approximately 60 times the maximum recommended human dose based on surface area). Glimepiride has been shown to be associated with intrauterine fetal death in rats when given in doses as low as 50 times the human dose based on surface area and in rabbits when given in doses as low as 0.1 times the human dose based on surface area. This fetotoxicity, observed only at doses inducing maternal hypoglycemia, has been similarly noted with other sulfonylureas, and is believed to be directly related to the pharmacologic (hypoglycemic) action of glimepiride.
There are no adequate and well-controlled studies in pregnant women. On the basis of results from animal studies, glimepiride tablets should not be used during pregnancy. Because recent information suggests that abnormal blood glucose levels during pregnancy are associated with a higher incidence of congenital abnormalities, many experts recommend that insulin be used during pregnancy to maintain glucose levels as close to normal as possible.
In some studies in rats, offspring of dams exposed to high levels of glimepiride during pregnancy and lactation developed skeletal deformities consisting of shortening, thickening, and bending of the humerus during the postnatal period. Significant concentrations of glimepiride were observed in the serum and breast milk of the dams as well as in the serum of the pups. These skeletal deformations were determined to be the result of nursing from mothers exposed to glimepiride.
Prolonged severe hypoglycemia (4 to 10 days) has been reported in neonates born to mothers who were receiving a sulfonylurea drug at the time of delivery. This has been reported more frequently with the use of agents with prolonged half-lives. Patients who are planning a pregnancy should consult their physician, and it is recommended that they change over to insulin for the entire course of pregnancy and lactation.
In rat reproduction studies, significant concentrations of glimepiride were observed in the serum and breast milk of the dams, as well as in the serum of the pups. Although it is not known whether glimepiride is excreted in human milk, other sulfonylureas are excreted in human milk. Because the potential for hypoglycemia in nursing infants may exist, and because of the effects on nursing animals, glimepiride should be discontinued in nursing mothers. If glimepiride is discontinued, and if diet and exercise alone are inadequate for controlling blood glucose, insulin therapy should be considered. (See above Pregnancy, Nonteratogenic Effects.)
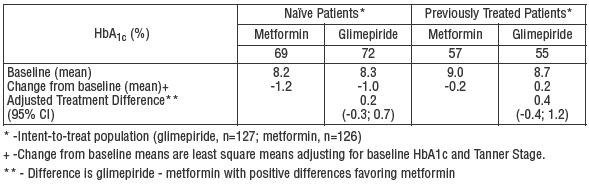
The safety and efficacy of glimepiride were evaluated in an active-controlled, single-blind (patients only), 24-week trial involving 272 pediatric patients, ranging from 8 to 17 years of age, with Type 2 diabetes. Glimepiride (n=135) was administered at 1 mg initially, and then titrated up to 2, 4 or 8 mg (mean last dose 4 mg) until the therapeutic goal of self-monitored fasting blood glucose < 7.0 mmol/L (< 126 mg/dL) was achieved. The active comparator metformin (n=137) was administered at 500 mg twice daily initially and titrated up to 1000 mg twice daily (mean last dose 1365 mg).
In US clinical studies of glimepiride, 608 of 1986 patients were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Comparison of glimepiride pharmacokinetics in Type 2 diabetic patients ≤65 years (n=49) and those >65 years (n=42) was performed in a study using a dosing regimen of 6 mg daily. There were no significant differences in glimepiride pharmacokinetics between the two age groups (see CLINICAL PHARMACOLOGY, Special Populations, Geriatric).
The drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Elderly patients are particularly susceptible to hypoglycemic action of glucose-lowering drugs. In elderly, debilitated, or malnourished patients, or in patients with renal and hepatic insufficiency, the initial dosing, dose increments, and maintenance dosage should be conservative based upon blood glucose levels prior to and after initiation of treatment to avoid hypoglycemic reactions. Hypoglycemia may be difficult to recognize in the elderly and in people who are taking beta-adrenergic blocking drugs or other sympatholytic agents (see CLINICAL PHARMACOLOGY, Special Populations, Renal Insufficiency; PRECAUTIONS, General; and DOSING AND ADMINISTRATION, Special Patient Population).
GLIMEPIRIDE ADVERSE REACTIONS
Adult Patients
The incidence of hypoglycemia with glimepiride, as documented by blood glucose values <60 mg/dL, ranged from 0.9-1.7% in two large, well-controlled, 1-year studies. (See WARNINGS and PRECAUTIONS.)
Glimepiride has been evaluated for safety in 2,013 patients in US controlled trials, and in 1,551 patients in foreign controlled trials. More than 1,650 of these patients were treated for at least 1 year.
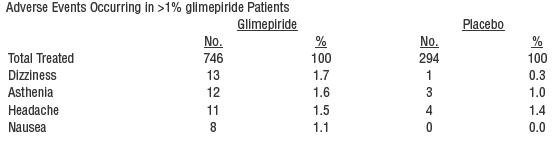
Adverse events, other than hypoglycemia, considered to be possibly or probably related to study drug that occurred in US placebo-controlled trials in more than 1% of patients treated with glimepiride are shown below.
Gastrointestinal Reactions
Vomiting, gastrointestinal pain, and diarrhea have been reported, but the incidence in placebo-controlled trials was less than 1%. In rare cases, there may be an elevation of liver enzyme levels. In isolated instances, impairment of liver function (e.g. with cholestasis and jaundice), as well as hepatitis, which may also lead to liver failure have been reported with sulfonylureas, including glimepiride.
Dermatologic Reactions
Allergic skin reactions, e.g., pruritus, erythema, urticaria, and morbilliform or maculopapular eruptions, occur in less than 1% of treated patients. These may be transient and may disappear despite continued use of glimepiride. If those hypersensitivity reactions persist or worsen (e.g., dyspnea, fall in blood pressure, shock), the drug should be discontinued.
Porphyria cutanea tarda, photosensitivity reactions, and allergic vasculitis have been reported with sulfonylureas, including glimepiride.
Hematologic Reactions
Leukopenia, agranulocytosis, thrombocytopenia, hemolytic anemia, aplastic anemia, and pancytopenia have been reported with sulfonylureas, including glimepiride.
Metabolic Reactions
Hepatic porphyria reactions and disulfiram-like reactions have been reported with sulfonylureas, including glimepiride. Cases of hyponatremia have been reported with glimepiride and all other sulfonylureas, most often in patients who are on other medications or have medical conditions known to cause hyponatremia or increase release of antidiuretic hormone. The syndrome of inappropriate antidiuretic hormone (SIADH) secretion has been reported with sulfonylureas, including, glimepiride and it has been suggested that certain sulfonylureas may augment the peripheral (antidiuretic) action of ADH and/or increase release of ADH.
Other Reactions
Changes in accommodation and/or blurred vision may occur with the use of glimepiride. This is thought to be due to changes in blood glucose, and may be more pronounced when treatment is initiated. This condition is also seen in untreated diabetic patients, and may actually be reduced by treatment. In placebo-controlled trials of glimepiride, the incidence of blurred vision was placebo, 0.7%, and glimepiride, 0.4%.
Pediatric Patients
In a clinical trial, 135 pediatric patients with Type 2 diabetes were treated with glimepiride. The profile of adverse reactions in these patients was similar to that observed in adults.
OVERDOSAGE
Overdosage of sulfonylureas, including glimepiride, can produce hypoglycemia. Mild hypoglycemic symptoms without loss of consciousness or neurologic findings should be treated aggressively with oral glucose and adjustments in drug dosage and/or meal patterns. Close monitoring should continue until the physician is assured that the patient is out of danger. Severe hypoglycemic reactions with coma, seizure, or other neurological impairment occur infrequently, but constitute medical emergencies requiring immediate hospitalization. If hypoglycemic coma is diagnosed or suspected, the patient should be given a rapid intravenous injection of concentrated (50%) glucose solution. This should be followed by a continuous infusion of a more dilute (10%) glucose solution at a rate that will maintain the blood glucose at a level above 100 mg/dL. Patients should be closely monitored for a minimum of 24 to 48 hours, because hypoglycemia may recur after apparent clinical recovery.
GLIMEPIRIDE DOSAGE AND ADMINISTRATION
There is no fixed dosage regimen for the management of diabetes mellitus with glimepiride or any other hypoglycemic agent. The patient’s fasting blood glucose and HbA1c must be measured periodically to determine the minimum effective dose for the patient; to detect primary failure, i.e., inadequate lowering of blood glucose at the maximum recommended dose of medication; and to detect secondary failure, i.e., loss of adequate blood glucose lowering response after an initial period of effectiveness. Glycosylated hemoglobin levels should be performed to monitor the patient’s response to therapy.
Short-term administration of glimepiride may be sufficient during periods of transient loss of control in patients usually controlled well on diet and exercise.
The usual starting dose of glimepiride as initial therapy is 1-2 mg once daily, administered with breakfast or the first main meal. Those patients who may be more sensitive to hypoglycemic drugs should be started at 1 mg once daily, and should be titrated carefully. (See PRECAUTIONS Section for patients at increased risk.)
No exact dosage relationship exists between glimepiride and the other oral hypoglycemic agents. The maximum starting dose of glimepiride should be no more than 2 mg.
Failure to follow an appropriate dosage regimen may precipitate hypoglycemia. Patients who do not adhere to their prescribed dietary and drug regimen are more prone to exhibit unsatisfactory response to therapy.
The usual maintenance dose is 1 to 4 mg once daily. The maximum recommended dose is 8 mg once daily. After reaching a dose of 2 mg, dosage increases should be made in increments of no more than 2 mg at 1-2 week intervals based upon the patient’s blood glucose response. Long-term efficacy should be monitored by measurement of HbA1c levels, for example, every 3 to 6 months.
If patients do not respond adequately to the maximal dose of glimepiride monotherapy, addition of metformin may be considered. Published clinical information exists for the use of other sulfonylureas including glyburide, glipizide, chlorpropamide, and tolbutamide in combination with metformin.
With concomitant glimepiride and metformin therapy, the desired control of blood glucose may be obtained by adjusting the dose of each drug. However, attempts should be made to identify the minimum effective dose of each drug to achieve this goal. With concomitant glimepiride and metformin therapy, the risk of hypoglycemia associated with glimepiride therapy continues and may be increased. Appropriate precautions should be taken.
Combination therapy with glimepiride and insulin may also be used in secondary failure patients. The fasting glucose level for instituting combination therapy is in the range of >150 mg/dL in plasma or serum depending on the patient. The recommended glimepiride dose is 8 mg once daily administered with the first main meal. After starting with low-dose insulin, upward adjustments of insulin can be done approximately weekly as guided by frequent measurements of fasting blood glucose. Once stable, combination-therapy patients should monitor their capillary blood glucose on an ongoing basis, preferably daily. Periodic adjustments of insulin may also be necessary during maintenance as guided by glucose and HbA1c levels.
Glimepiride tablets are not recommended for use in pregnancy or nursing mothers. Data are insufficient to recommend pediatric use of glimepiride. In elderly, debilitated, or malnourished patients, or in patients with renal or hepatic insufficiency, the initial dosing, dose increments, and maintenance dosage should be conservative to avoid hypoglycemic reactions (See CLINICAL PHARMACOLOGY, Special Populations and PRECAUTIONS, General).
As with other sulfonylurea hypoglycemic agents, no transition period is necessary when transferring patients to glimepiride. Patients should be observed carefully (1-2 weeks) for hypoglycemia when being transferred from longer half-life sulfonylureas (e.g., chlorpropamide) to glimepiride due to potential overlapping of drug effect.
HOW SUPPLIED
Glimepiride Tablets, USP are available in the following strengths and package sizes:
1 mg (pink, round, flat faced, beveled edge tablets de-bossed with I on the left side of bisect and G on right side of bisect on one side and “203” on the other).
Bottles of 100 NDC 45802-770-78
2 mg (green, round, flat faced, beveled edge tablets de-bossed with I on the left side of bisect and G on right side of bisect on one side and “204” on the other).
Bottles of 100 NDC 45802-822-78
4 mg (blue, round, flat faced, beveled edge tablets de-bossed with I on the left side of bisect and G on right side of bisect on one side and “205” on the other).
Bottles of 100 NDC 45802-947-78
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
Dispense in well-closed containers with safety closures.
ANIMAL TOXICOLOGY
Reduced serum glucose values and degranulation of the pancreatic beta cells were observed in beagle dogs exposed to 320 mg glimepiride/kg/day for 12 months (approximately 1,000 times the recommended human dose based on surface area). No evidence of tumor formation was observed in any organ. One female and one male dog developed bilateral subcapsular cataracts. Non-GLP studies indicated that glimepiride was unlikely to exacerbate cataract formation. Evaluation of the cocataractogenic potential of glimepiride in several diabetic and cataract rat models was negative and there was no adverse effect of glimepiride on bovine ocular lens metabolism in organ culture.
HUMAN OPHTHALMOLOGY DATA
Ophthalmic examinations were carried out in over 500 subjects during long-term studies using the methodology of Taylor and West and Laties et al. No significant differences were seen between glimepiride and glyburide in the number of subjects with clinically important changes in visual acuity, intra-ocular tension, or in any of the five lens-related variables examined.
Ophthalmic examinations were carried out during long-term studies using the method of Chylack et al. No significant or clinically meaningful differences were seen between glimepiride and glipizide with respect to cataract progression by subjective LOCS II grading and objective image analysis systems, visual acuity, intraocular pressure, and general ophthalmic examination.
Manufactured by:
InvaGen Pharmaceuticals, Inc.
Hauppauge, NY 11788
DISTRIBUTED BY
PERRIGO®
ALLEGAN, MI 49010
OH700 RC J4
Rev: 08/10
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Glimepiride Tablets, USP 4 mg
Once a Day
Rx Only
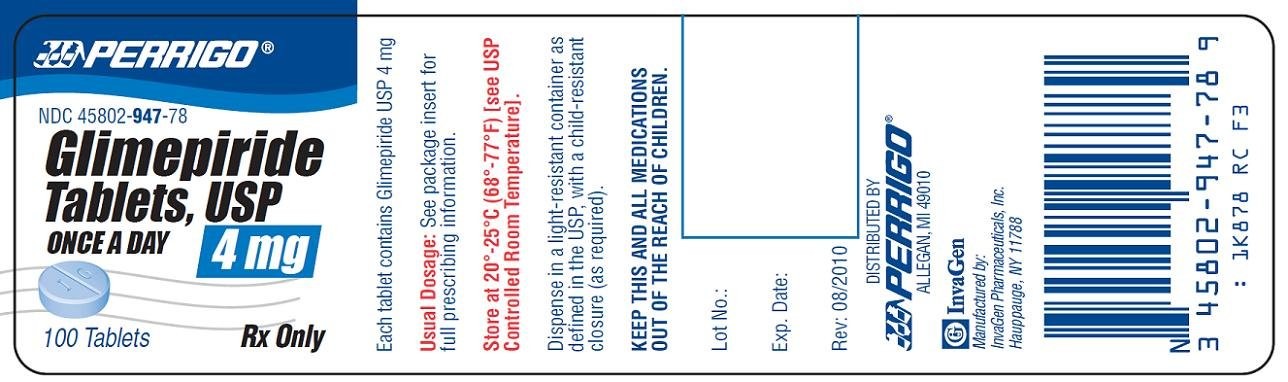
GlimepirideGlimepiride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||