GENTACALM
Dechra Veterinary Products
American Pharmaceuticals and Cosmetics, Inc.
GENTACALM Topical Spray
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Front Panel Label
GENTACALM
Topical Spray
(Gentamicin Sulfate With
Betamethasone Valerate)
Veterinary
CAUTION: Federal Law restricts this drug
to use by or on the order of a
licensed veterinarian
ANADA 200-388, Approved by FDA
NDC 17033-335-12
Net Contents: 60 mL, 120 mL, or 240 mL Bottles
For topical use in dogs only.
For animal use only.
Keep out of Reach of Children.
Usual dose:
Read accompanying directions carefully.
00
00
PRODUCT INFORMATION
ANADA 200-388, Approved by FDA
GENTAMICIN SULFATE WITH BETAMETHASONE
VALERATE TOPICAL SPRAY
(Gentamicin Sulfate, USP With Betamethasone
Valerate, USP Topical Spray)
Veterinary
For Topical Use in Dogs Only
For Animal Use Only
to use by or on the order of a
licensed veterinarian
DESCRIPTION:
CHEMISTRY: Gentamicin is a mixture of aminoglycoside antibiotics derived from the
fermentation of Micromonospora purpurea. Gentamicin sulfate is a mixture of sulfate
salts of the antibiotics produced in this fermentation. The salts are weakly acidic
and freely soluble in water.
Gentamicin sulfate contains not less than 500 micrograms of gentamicin base per
milligram.
Betamethasone valerate is a synthetic glucocorticoid.
PHARMACOLOGY: Gentamicin, a broad-spectrum antibiotic, is a highly
effective topical treatment for bacterial infection of the skin In vitro,
gentamicin is bactericidal against a wide variety of gram-positive and gram-
negative bacteria isolated from domestic animals. 1,2 Specifically, gentamicin is
active against the following organisms isolated from canine skin: Alcaligenes
sp., Citrobacter sp., Klebsiella sp., Pseudomonas aeruginosa, indole-positive
and -negative Proteus sp., Escherichia coli, Enterobacter sp., Staphlyococcus sp.
and Streptococcus sp.
Betamethasone valerate emerged from intensive research as the most
promising of some 50 newly synthesized corticosteroids in the
experimental model described by McKenzie,3 et al. This human bioassay
technique has been found reliable for evaluating vasoconstrictor
properties of new topical corticosteroids and is useful in predicting clinical
efficacy.
Betamethasone valerate in veterinary medicine has been shown to provide
anti-inflammatory and antipruritic activity in the topical management of
corticosteroid-responsive infected superficial lesions in dogs.
WARNING:
Uses
INDICATIONS: For the treatment of infected superficial lesions in dogs
caused by bacteria susceptible to gentamicin.
CONTRAINDICATIONS: If hypersensitivity of any of the components
occurs, discontinue treatment and institute appropriate therapy.
DOSAGE AND ADMINISTRATION: Prior to treatment, remove excessive
hair and clean the lesion and adjacent area. Hold bottle upright 3 to 6
inches from the lesion and depress the sprayer head twice. Administer 2
to 4 times daily for 7 days.
Each depression of the sprayer head delivers 0.7 mL of Gentamicin
Sulfate With Betamethasone Valerate Topical Spray.
SIDE EFFECTS:
PRECAUTIONS:
HOW SUPPLIED:
Gentamicin Sulfate with Betamethasone Valerate Topical Spray.
Store upright between 20C and 300C (360F and 860F).REFERENCES:
Gentacalm 60 mL Bottle Label
60 mL Bottle Label

60 mL box image
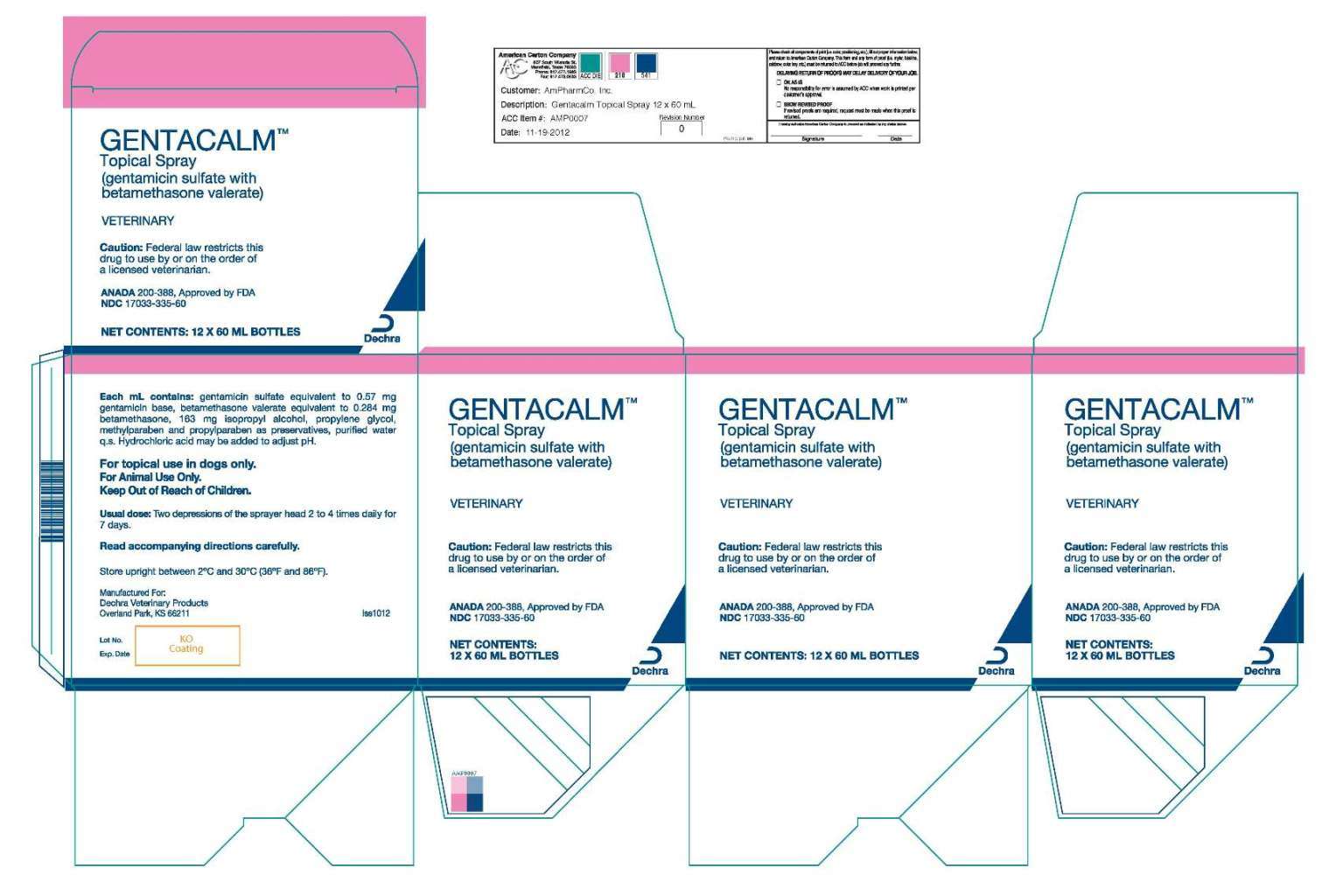
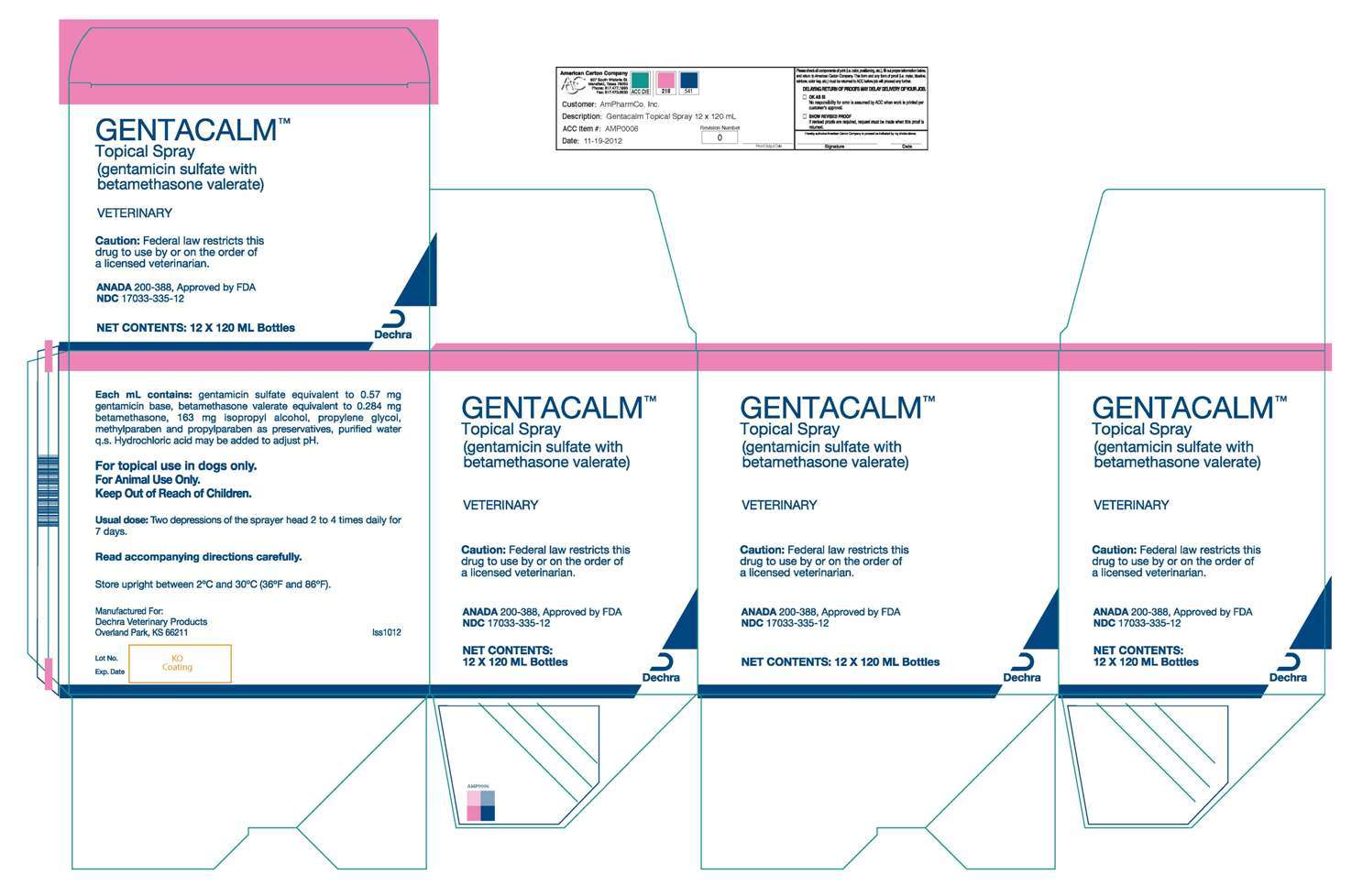
120 mL bottle label image

120 mL box image
240 mL bottle image label

240 mL box image

Outcert Front Panel
Outcert front panel

Outcert Back Panel
Outcert back panel

GENTACALMGentamicin sulfate and betamethasone valerate SPRAY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||