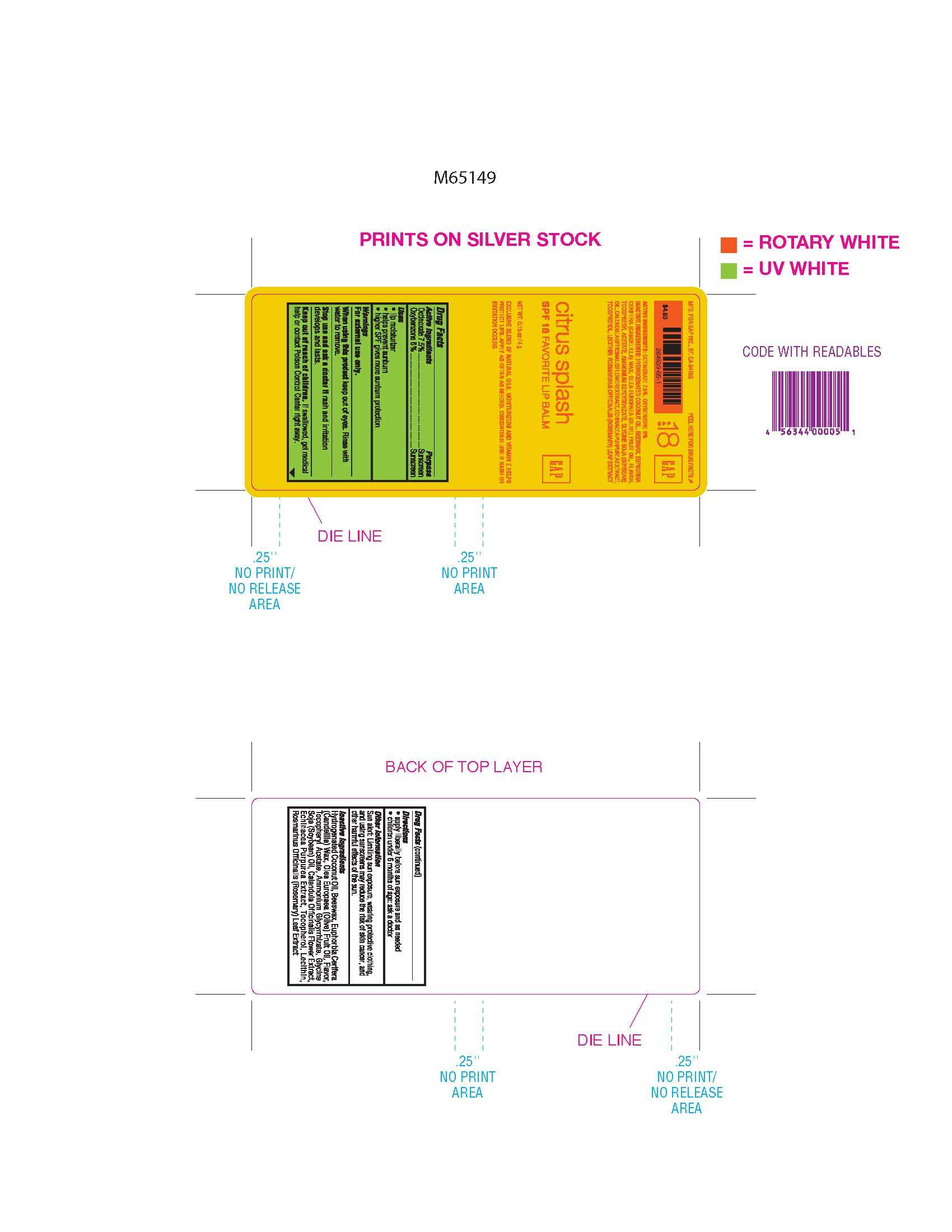
GAP Favorite Lip Balm
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Warning
- When using this product
- Stop use
- Keep out of reach of children
- Directions
- Sun Alert
- Inactive Ingredients
FULL PRESCRIBING INFORMATION
Active Ingredients
Active Ingredients Purpose
Octinoxate 7.5% Sunscreen
Oxybenzone 6% Sunscreen
Purpose
- lip moisturizer
- helps prevent sunburn
- higher SPF gives more sunburn protection
Warning
Warnings
For external use only
When using this product
When using this product keep out of eyes. Rinse with water to remove
Stop use
Stop use and ask a doctor if rash and irritation develops and lasts
Keep out of reach of children
Keep out of reach of children. If swallowed get medical help or contact Poison Control right away.
Directions
Directions
- apply liberally before sun exposure and as needed
- children under 6 months of age: ask doctor
Sun Alert
Other information
Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreen may reduce the risk of skin cancer, and other harmful effects of the sun.
Inactive Ingredients
Inactive Ingredients: Hydrogenated Coconut Oil, Beeswax, Euphorbia Cerifera (Candelilla) Wax, Olea Europaea (Olive) Fruit Oil, Flavor, Tocopheryl Acetate, Ammonium Glycyrrhizate, Glycine Soja (Soybean) Oil, Calendula Officinalis Flower Extract, Echinacea Purpurea Extract, Tocopherol, Lecithin, Rosmarinus Officinalis (Rosemary) Leaf Extract.
GAP Favorite Lip BalmOctinoxate and Oxybenzone STICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||