Gabapentin
FULL PRESCRIBING INFORMATION: CONTENTS*
- GABAPENTIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- INDICATIONS & USAGE
- GABAPENTIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- GABAPENTIN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
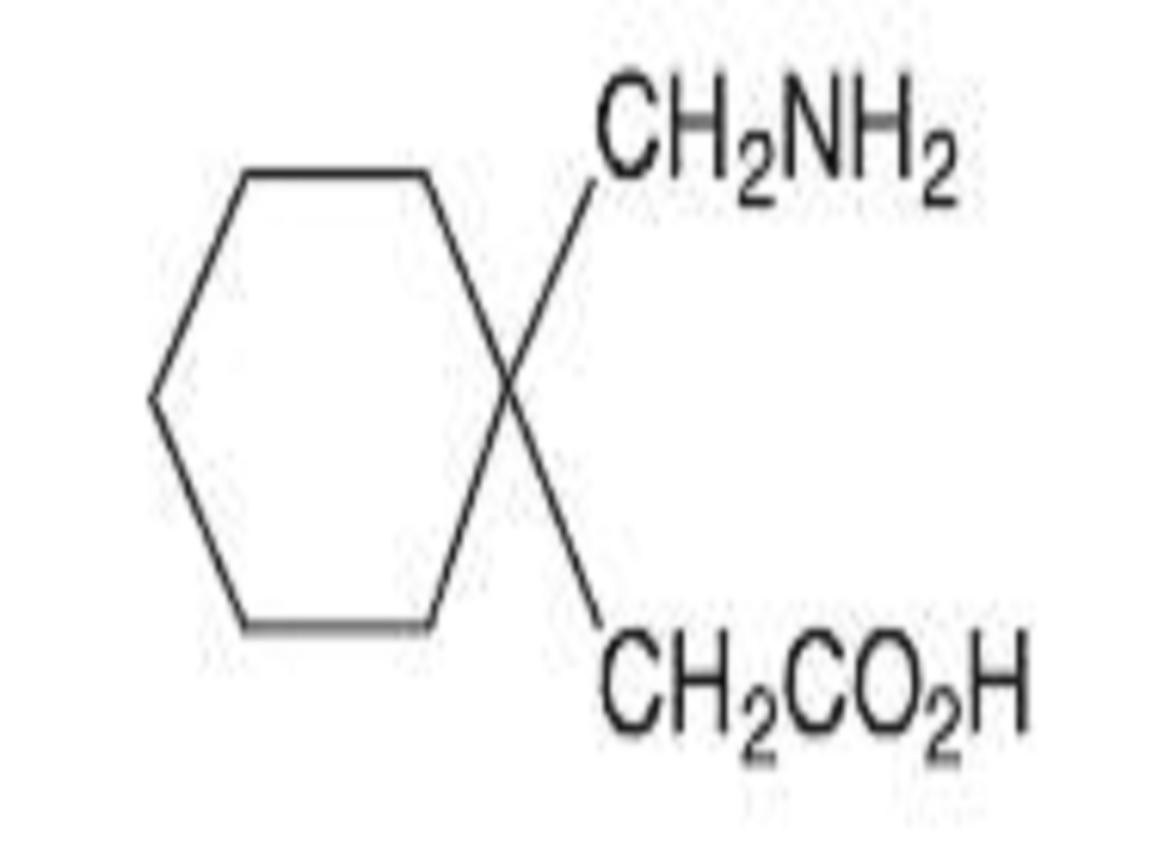
GABAPENTIN DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionPharmacokinetics and Drug Metabolism
Special Populations: Patients With Renal Insufficiency
DOSAGE AND ADMINISTRATION
Special Populations
Adult Patients With Renal Insufficiency
DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
PRECAUTIONSGeriatric UseDOSAGE AND ADMINISTRATION
Pediatric
DOSAGE AND ADMINISTRATION
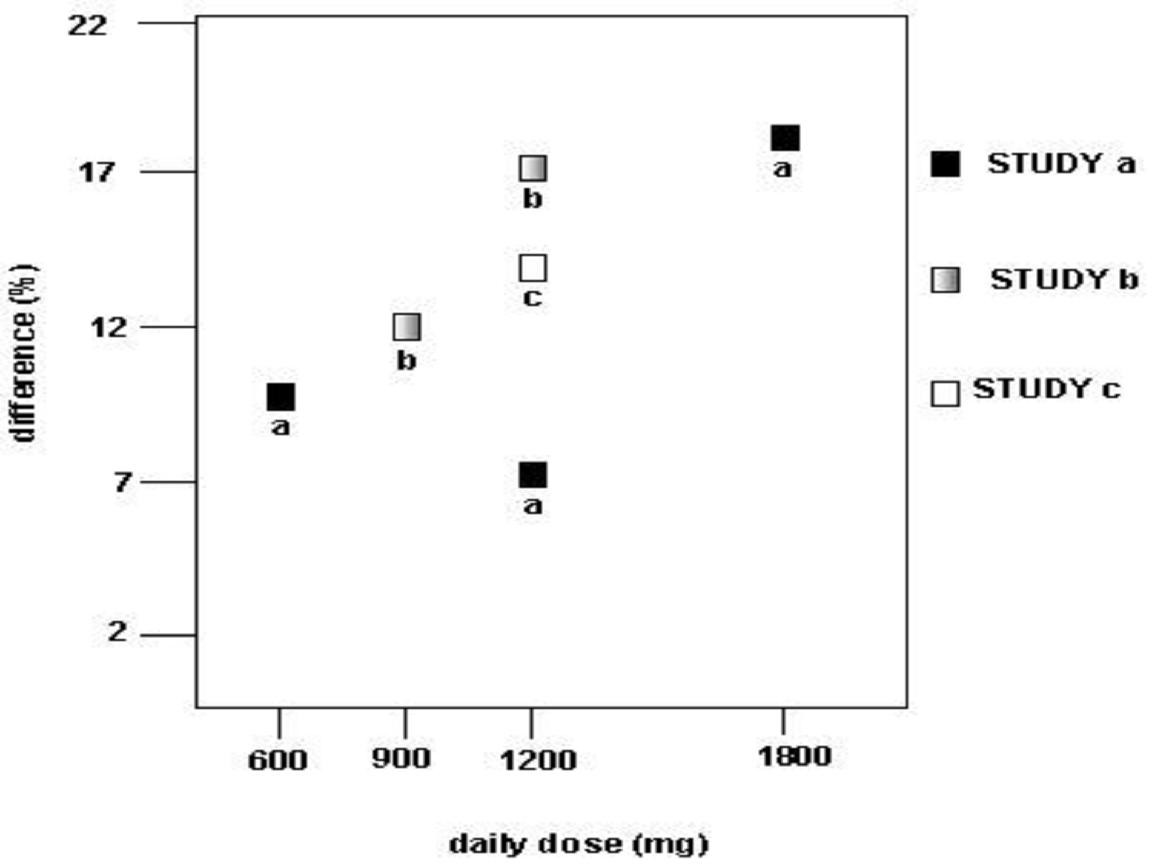
CLINICAL STUDIES
StudyStudy DurationGabapentin (mg/day)a Target DosePatients Receiving GabapentinPatients Receiving Placebo
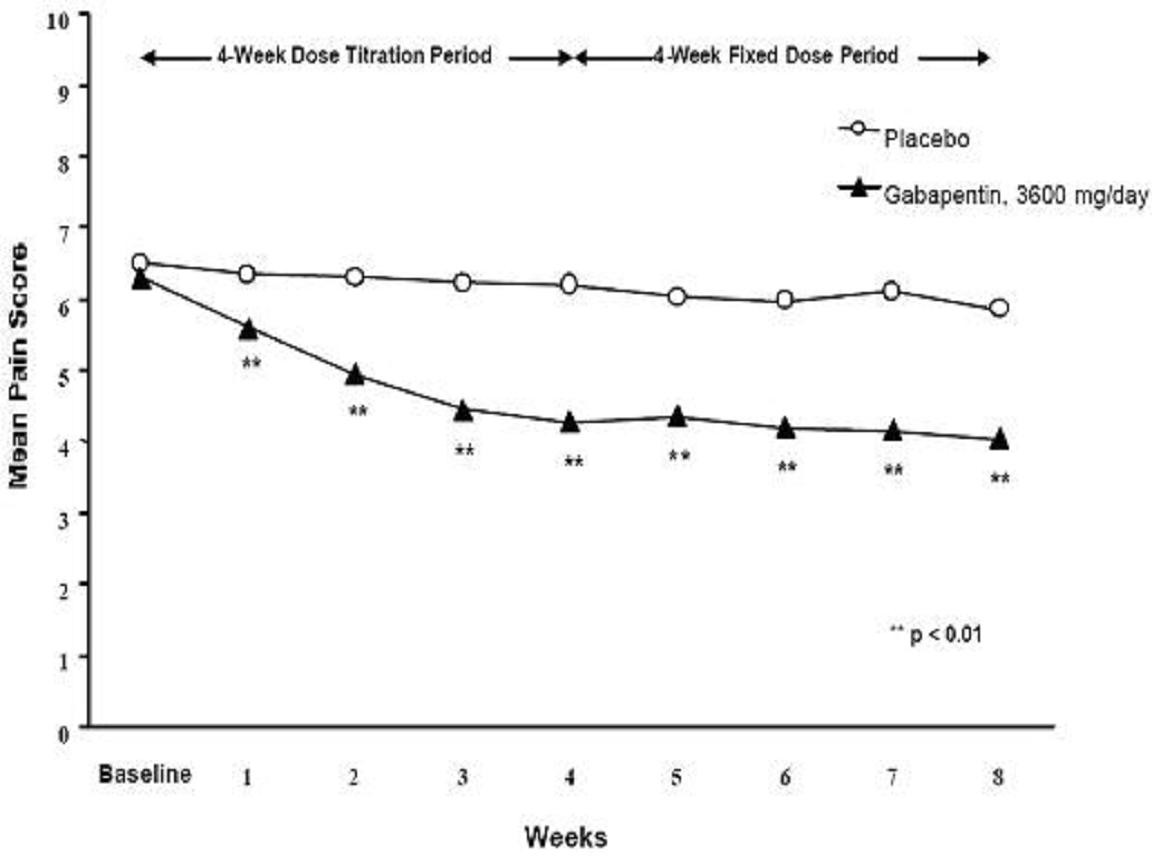
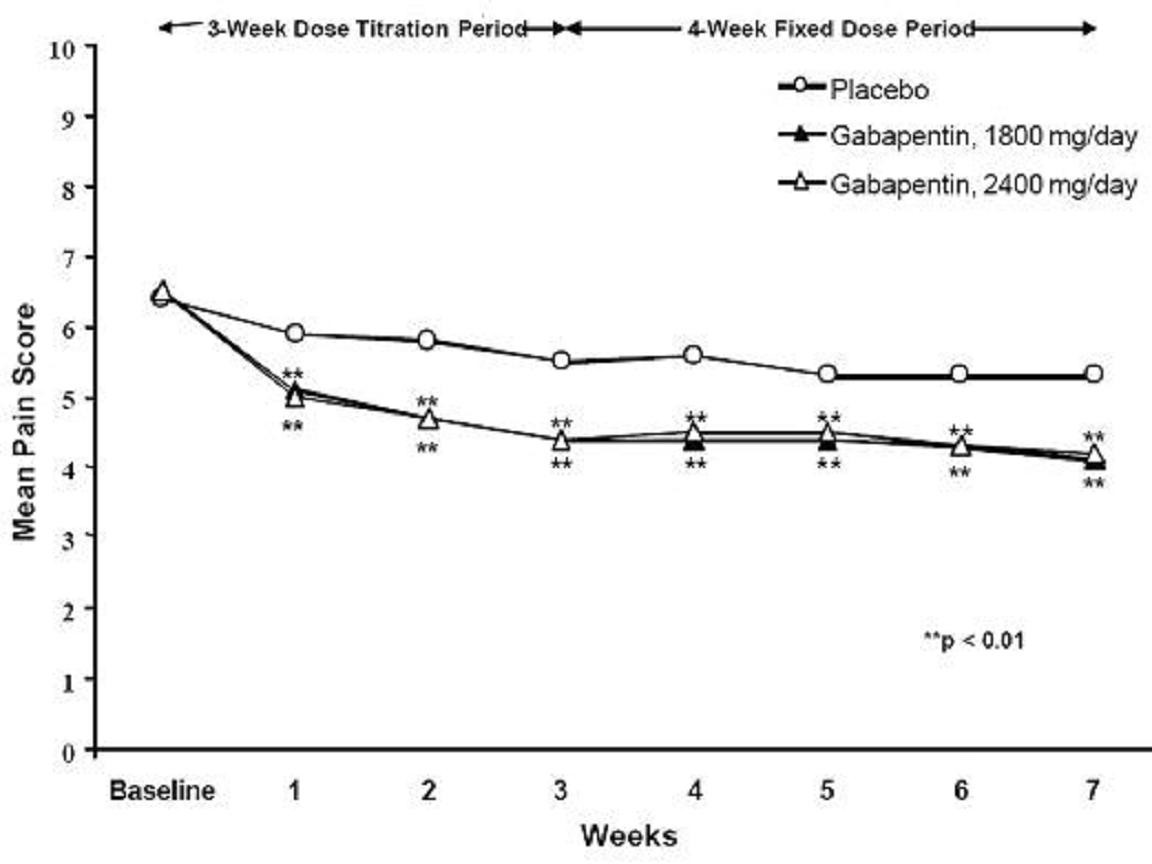
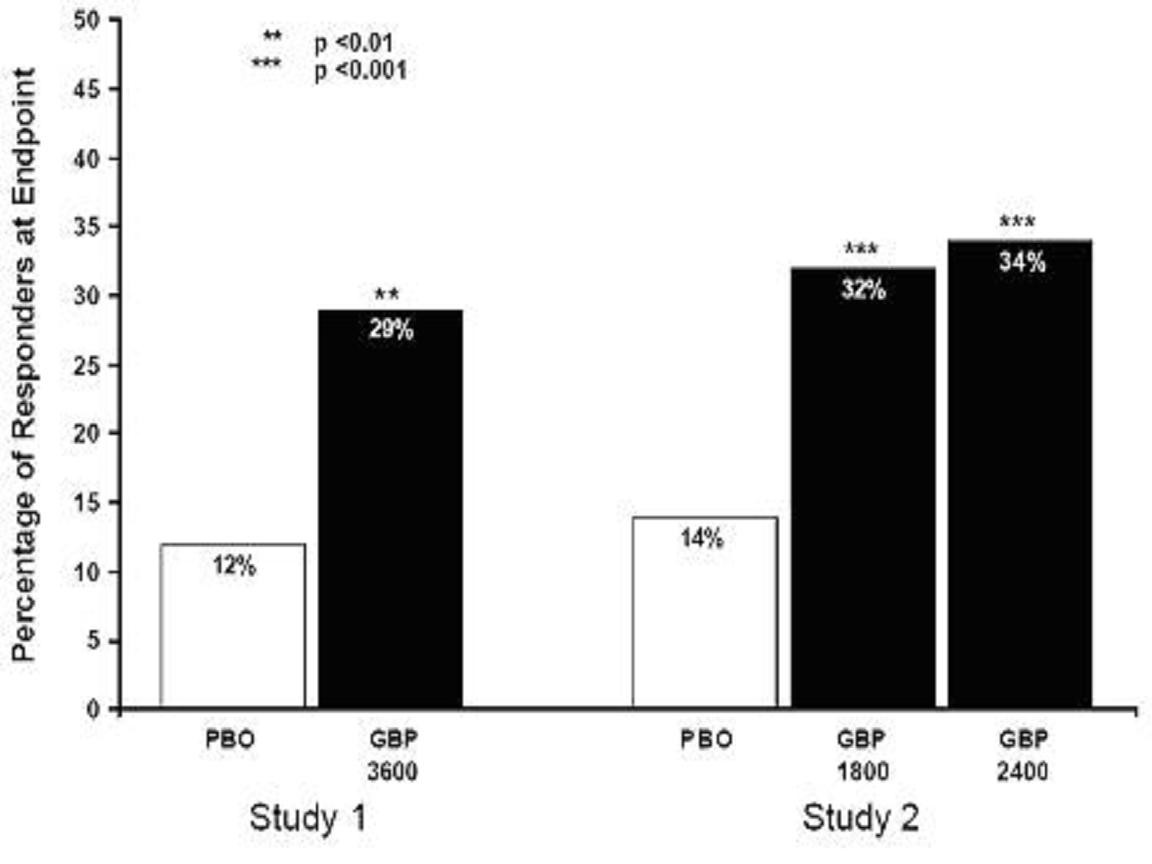
INDICATIONS & USAGE
Postherpetic NeuralgiaEpilepsy
GABAPENTIN CONTRAINDICATIONS
WARNINGS
Suicidal Behavior and IdeationIndicationPlacebo Patients with Events Per 1000 PatientsDrug Patients with Events Per 1000 PatientsRelative Risk: Incidence of Events in Drug Patients/Incidence in Placebo PatientsRisk Difference: Additional Drug Patients with Events Per 1000 Patients
Neuropsychiatric Adverse EventsPediatric Patients 3 to 12 years of age
Withdrawal Precipitated Seizure, Status Epilepticus
Tumorigenic Potential
PRECAUTIONS: Carcinogenesis, Mutagenesis, Impairment of Fertility
Sudden and Unexplained Death in Patients With Epilepsy
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
PRECAUTIONS
Information for PatientsDrug Interactions
PRECAUTIONS, Pregnancy
LABORATORY TESTS
DRUG INTERACTIONS
PRECAUTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsNURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGY, Clinical Studies
GERIATRIC USE
CLINICAL PHARMACOLOGYADVERSE REACTIONSDOSAGE AND ADMINISTRATION
GABAPENTIN ADVERSE REACTIONS
Postherpetic NeuralgiaIncidence in Controlled Clinical Trials
Body System/Preferred TermGabapentin N=336 %Placebo N=227 %
Epilepsy
WARNINGS , Neuropsychiatric Adverse Events
Incidence in Controlled Clinical Trials
Body System/Adverse EventGabapentina N=543 %Placeboa N=378 %
Body System/Adverse EventGabapentina N=119 %Placeboa N=128 %
Other Adverse Events Observed During All Clinical Trials
Body As A Whole:
Cardiovascular System:
Digestive System:
Endocrine System:
Hematologic and Lymphatic System:
Musculoskeletal System:
Nervous System:
Respiratory System:
Dermatological:
Urogenital System:
Special Senses:
Body as a Whole:
Digestive System:
Hemic and Lymphatic System:
Nervous System:
Psychobiologic Function:
Respiratory System:
Body as a Whole:
Cardiovascular System:
Digestive System:
Endocrine System:
Hemic and Lymphatic System:
Metabolic and Nutritional:
Musculoskeletal:
Nervous System:
Respiratory System:
Skin and Appendages:
Special Senses:
Urogenital System:
Postmarketing and Other Experience
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
DOSAGE & ADMINISTRATION
Postherpetic Neuralgia
Epilepsy
CLINICAL PHARMACOLOGY, Pediatrics
Dosage in Renal Impairment
Renal Function Creatinine Clearance (mL/min)Total Daily Dose Range (mg/day)Dose Regimen (mg)
Dosage in Elderly
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
Gabapentin Tablets, USPRx only
What is the most important information I should know about gabapentin tablets?
Do not stop taking gabapentin tablets without first talking to your healthcare provider.
Gabapentin tablets can cause serious side effects including:
1. Like other antiepileptic drugs, gabapentin tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
-
● Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
-
● Keep all follow-up visits with your healthcare provider as scheduled.
Do not stop taking gabapentin tablets without first talking to a healthcare provider.
-
● Stopping gabapentin tablets suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
2. Changes in behavior and thinking
3. Gabapentin tablets may cause a serious or life-threatening allergic reaction
-
● skin rash
-
● hives
-
● fever
-
● swollen glands that do not go away
-
● swelling of your lip and tongue
-
● yellowing of your skin or of the whites of the eyes
-
● unusual bruising or bleeding
-
● severe fatigue or weakness
-
● unexpected muscle pain
-
● frequent infections
What are gabapentin tablets?
-
● Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults.
-
● Partial seizures when taken together with other medicines in adults and children 3 years of age and older.
Who should not take gabapentin tablets?
What should I tell my healthcare provider before taking gabapentin tablets?
Before taking gabapentin tablets, tell your healthcare provider if you:
-
● have or have had kidney problems or are on hemodialysis
-
● have or have had depression, mood problems, or suicidal thoughts or behavior
-
● are pregnant or plan to become pregnant. It is not known if gabapentin tablets can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking gabapentin tablets. You and your healthcare provider will decide if you should take gabapentin tablets while you are pregnant.
-
● If you become pregnant while taking gabapentin tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy. You can enroll in this registry by calling 1-888-233-2334.
-
● are breastfeeding or plan to breastfeed. Gabapentin can pass into breast milk. You and your healthcare provider should decide how you will feed your baby while you take gabapentin tablets.
Tell your healthcare provider about all the medicines you take,
How should I take gabapentin tablets?
-
● Take gabapentin tablets exactly as prescribed. Your healthcare provider will tell you how much gabapentin tablets to take.
-
● Do not change your dose of gabapentin tablets without talking to your healthcare provider. If you break a tablet in half the unused half of the tablet should be taken at your next scheduled dose. Half tablets not used within several days of breaking should be thrown away.
-
● Gabapentin tablets can be taken with or without food. If you take an antacid containing aluminum and magnesium, such as MaaloxMylantaGelusilGavisconor Di-Gelyou should wait at least 2 hours before taking your next dose of gabapentin tablets.
-
● If you take too much gabapentin tablets, call your healthcare provider or your local Poison Control Center right away.
What should I avoid while taking gabapentin tablets?
-
● Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking gabapentin tablets without first talking with your healthcare provider. Taking gabapentin tablets with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
-
● Do not drive, operate heavy machinery, or do other dangerous activities until you know how gabapentin tablets affect you. Gabapentin tablets can slow your thinking and motor skills.
What are the possible side effects of gabapentin tablets?
-
● SeeWhat is the most important information I should know about gabapentin tablets?
-
● The most common side effects of gabapentin tablets include:
-
● dizziness
-
● difficulty with speaking
-
● lack of coordination
-
● temporary loss of memory (amnesia)
-
● viral infection
-
● tremor
-
● feeling drowsy
-
● difficulty with coordination
-
● feeling tired
-
● double vision
-
● fever
-
● unusual eye movement
-
● jerky movements
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store gabapentin tablets?
-
● Store gabapentin tablets between 20to 25(68to 77
Keep gabapentin tablets and all medicines out of the reach of children.
General information about the safe and effective use of gabapentin tablets
What are the ingredients in gabapentin tablets?
Active ingredient:
Inactive ingredients:
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

GabapentinGabapentin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!