Gabapentin
FULL PRESCRIBING INFORMATION: CONTENTS*
- GABAPENTIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- GABAPENTIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- GABAPENTIN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
GABAPENTIN DESCRIPTION

CLINICAL PHARMACOLOGY
PHARMACOKINETICS
Special Populations:Patients With Renal Insufficiency
DOSAGE AND ADMINISTRATION, Table 6
DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
PRECAUTIONS, Geriatric UseDOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
StudyStudyGabapentinPatients ReceivingPatients ReceivingDuration(mg/day)a TargetGabapentinPlaceboDoseTotal
INDICATIONS & USAGE
INDICATIONS & USAGEGABAPENTIN CONTRAINDICATIONS
CONTRAINDICATIONSWARNINGS
PRECAUTIONS: Carcinogenesis, Mutagenesis, Impairment of Fertility
PRECAUTIONS
Drug Interactions
PRECAUTIONS, Pregnancy
LABORATORY TESTS
DRUG INTERACTIONS
PRECAUTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
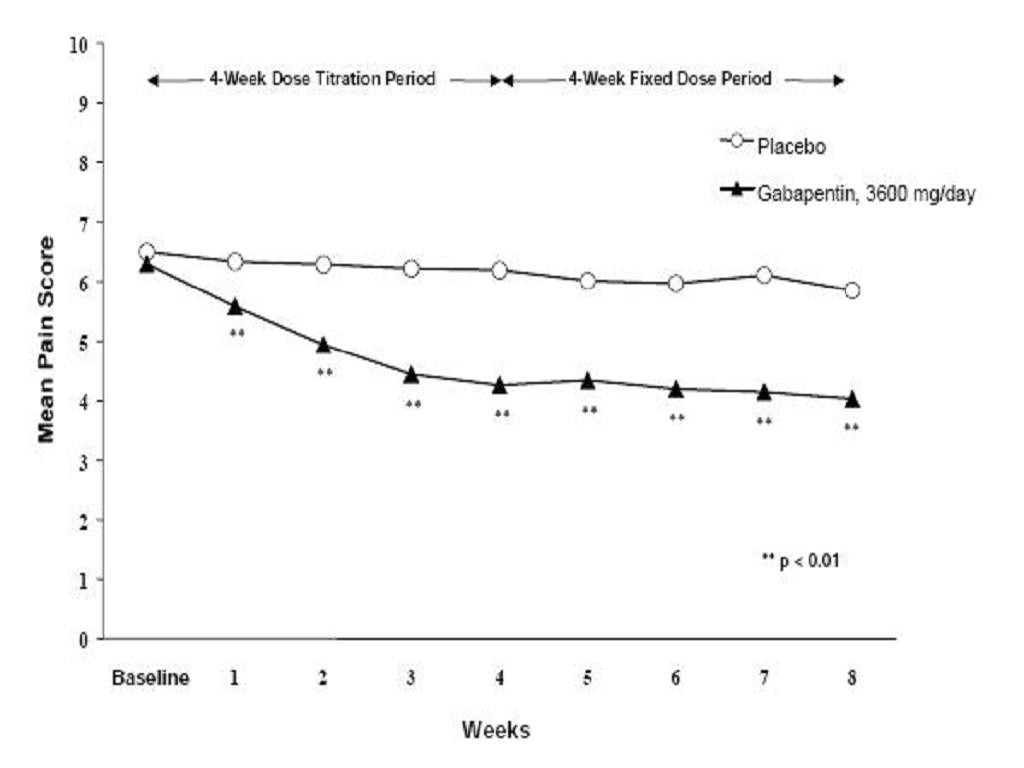
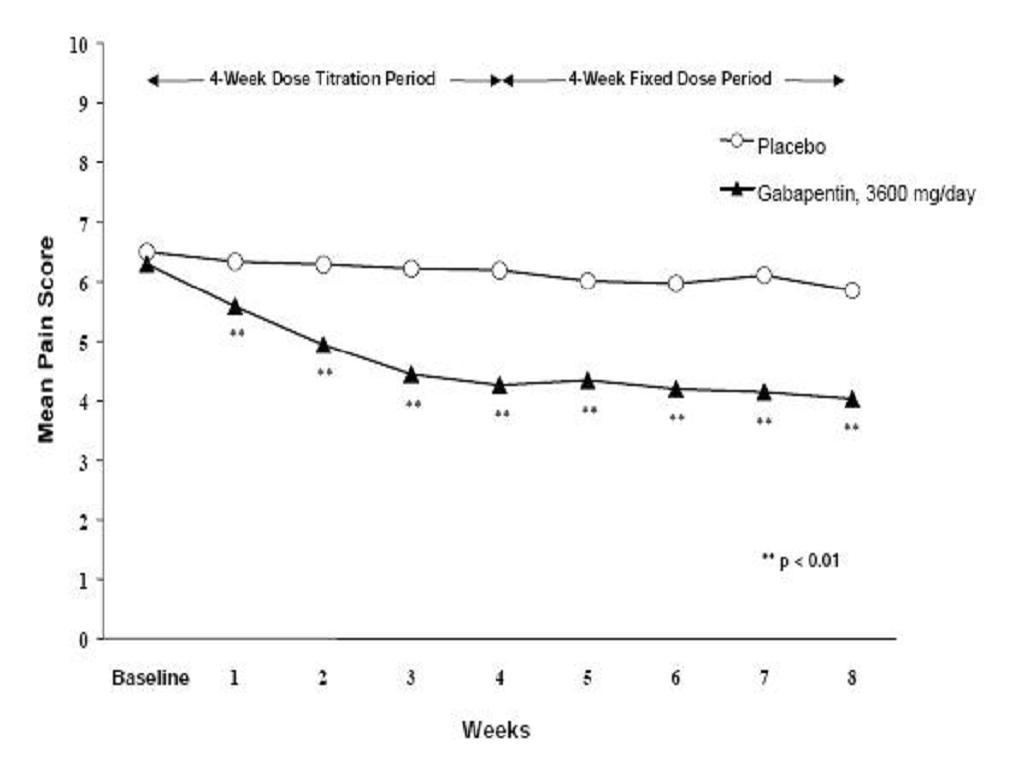
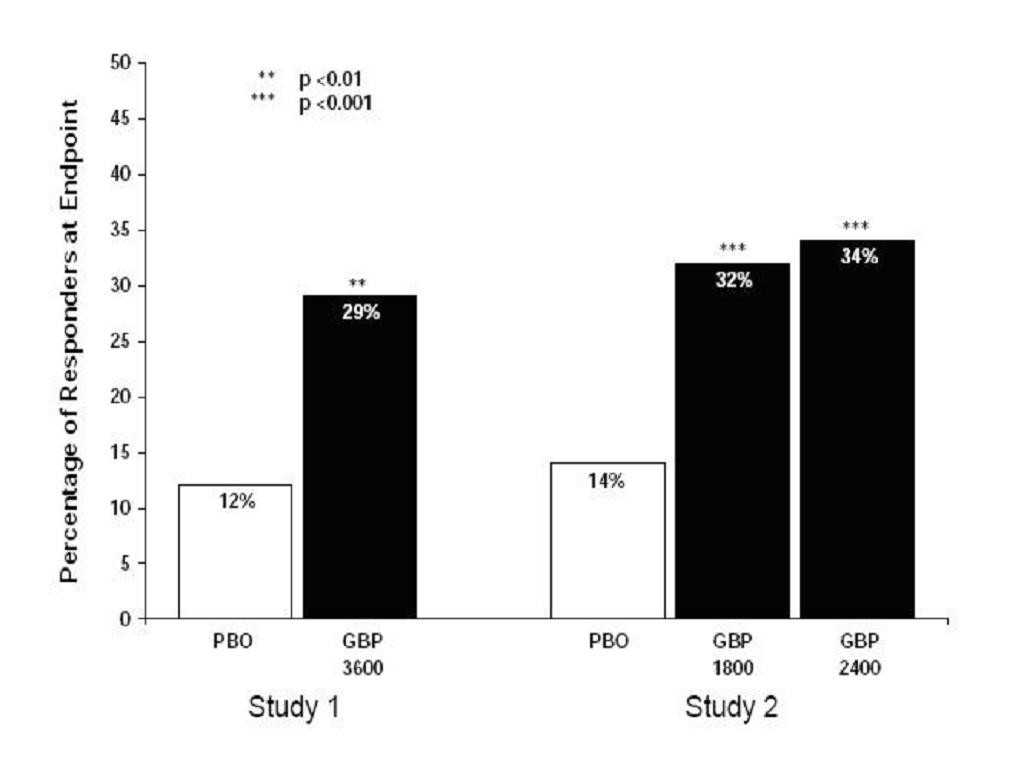
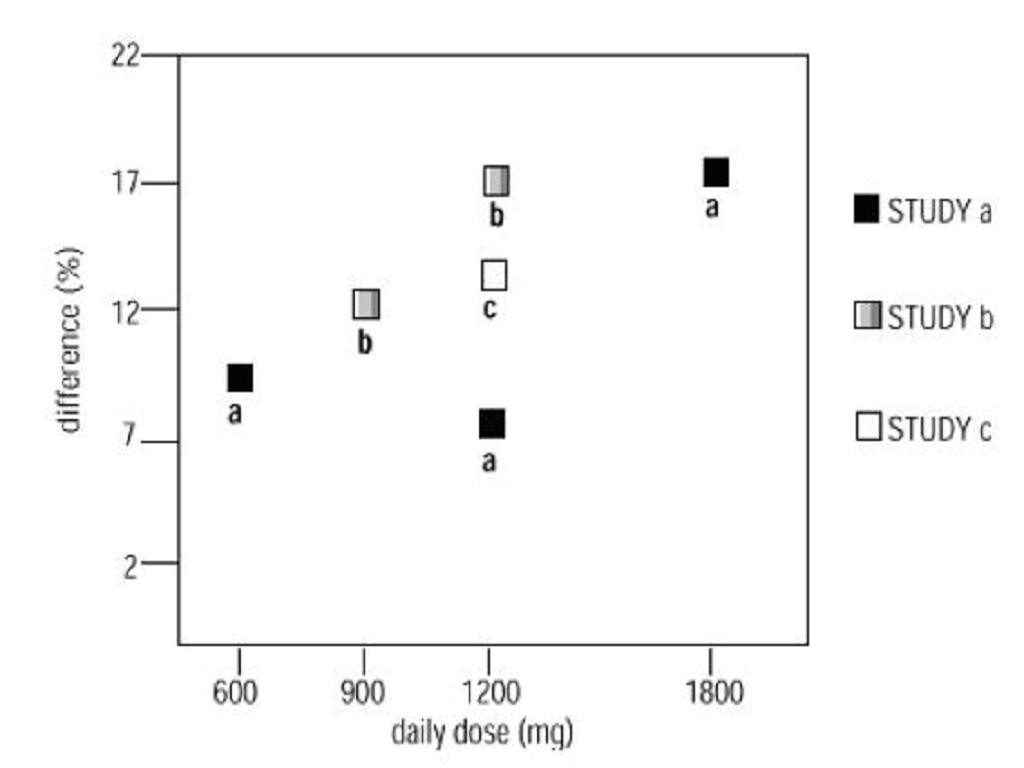
CLINICAL PHARMACOLOGY, Clinical Studies
GERIATRIC USE
CLINICAL PHARMACOLOGYADVERSE REACTIONSDOSAGE AND ADMINISTRATION
GABAPENTIN ADVERSE REACTIONS
Body System Preferred TermGabapentin CapsulesPlaceboPreferred TermN = 336N = 227%%Body as a WholeDigestive SystemMetabolic and Nutritional DisordersNervous SystemRespiratory SystemSkin and AppendagesSpecial Senses
WARNINGS, Neuropsychiatric Adverse Events
Body System /Gabapentin CapsulesaPlaceboaAdverse EventN = 543N = 378%%Body as a WholeCardiovascularDigestive SystemHematologic and Lymphatic SystemsMusculoskeletal SystemNervous SystemRespiratory SystemSkin and AppendagesUrogenital SystemSpecial SensesLaboratory Deviations
Body System/Gabapentin CapsulesaPlaceboaAdverse EventN = 119N = 128%%Body as a WholeDigestive SystemNervous SystemRespiratory System
DRUG ABUSE AND DEPENDENCE
DRUG ABUSE AND DEPENDENCEOVERDOSAGE
OVERDOSAGEDOSAGE & ADMINISTRATION
DOSAGE & ADMINISTRATIONCLINICAL PHARMACOLOGY, Pediatrics
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

GabapentinGabapentin CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!