Gabapentin
FULL PRESCRIBING INFORMATION: CONTENTS*
- GABAPENTIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS AND DRUG METABOLISM
- CLINICAL STUDIES
- INDICATIONS & USAGE
- GABAPENTIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- GABAPENTIN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
GABAPENTIN DESCRIPTION
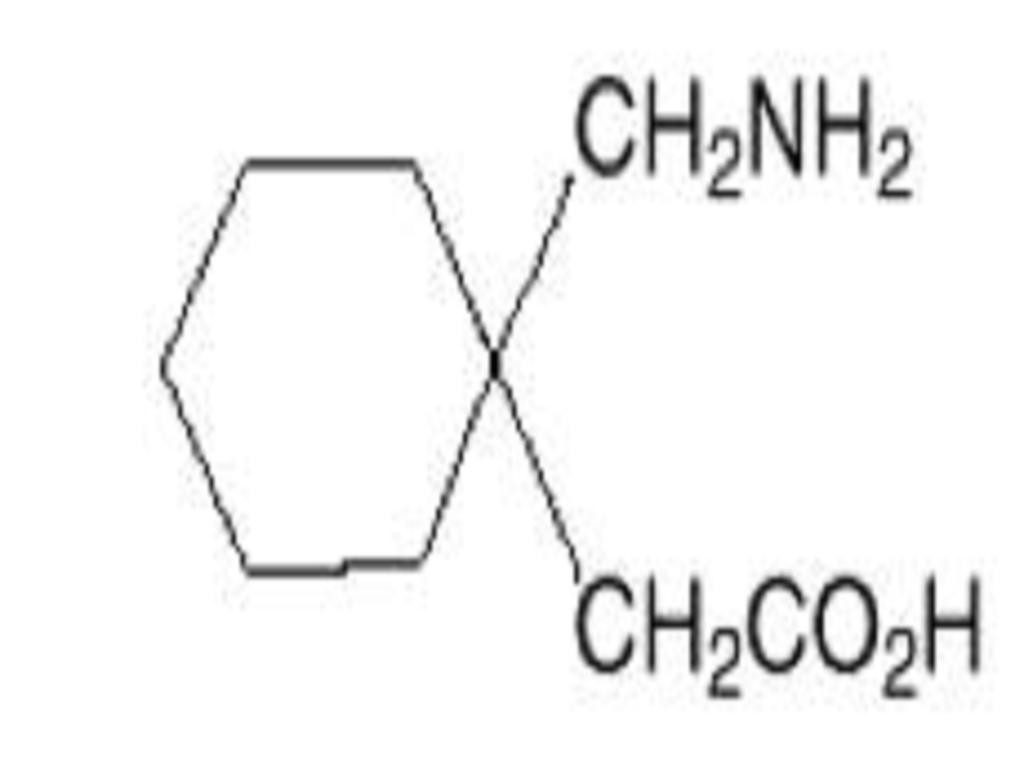
CLINICAL PHARMACOLOGY
Mechanism of ActionPHARMACOKINETICS AND DRUG METABOLISM
Special Populations:Patients With Renal Insufficiency
DOSAGE AND ADMINISTRATION, Table 6
DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
PRECAUTIONS, Geriatric UseDOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION
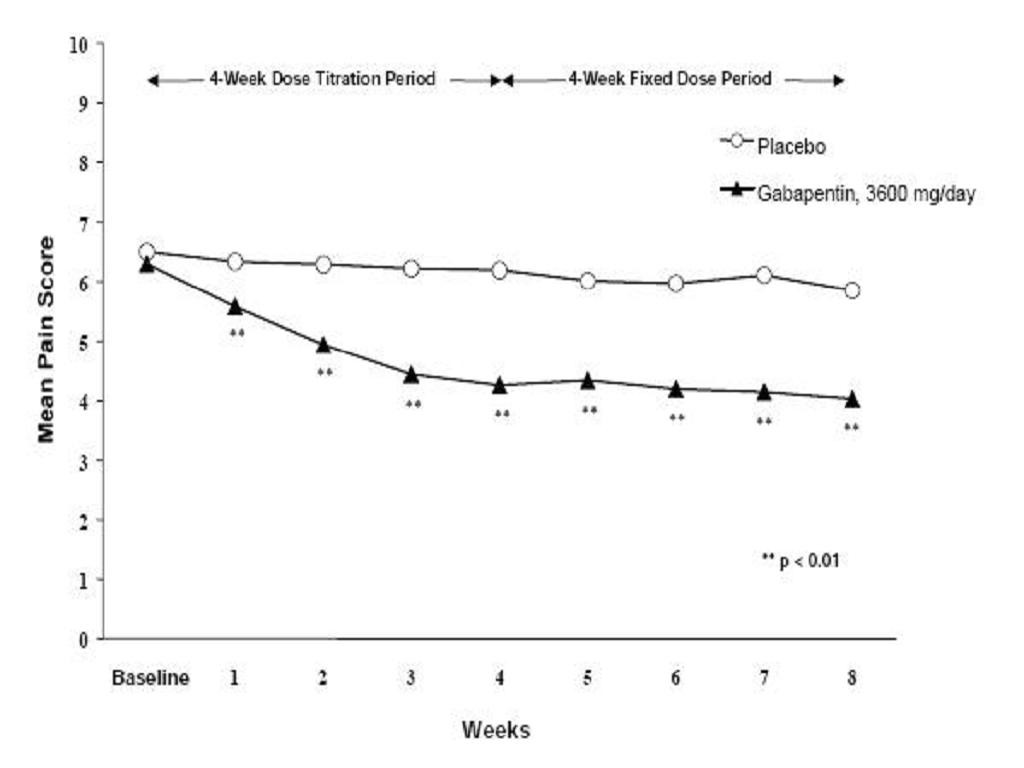
CLINICAL STUDIES
Postherpetic Neuralgia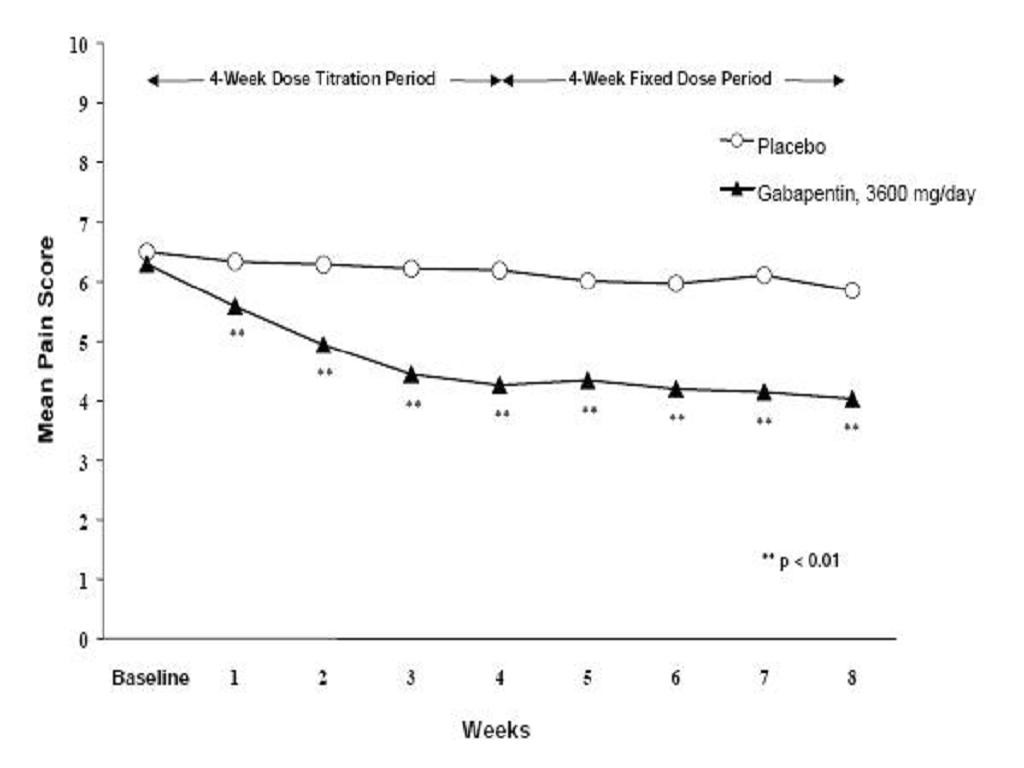
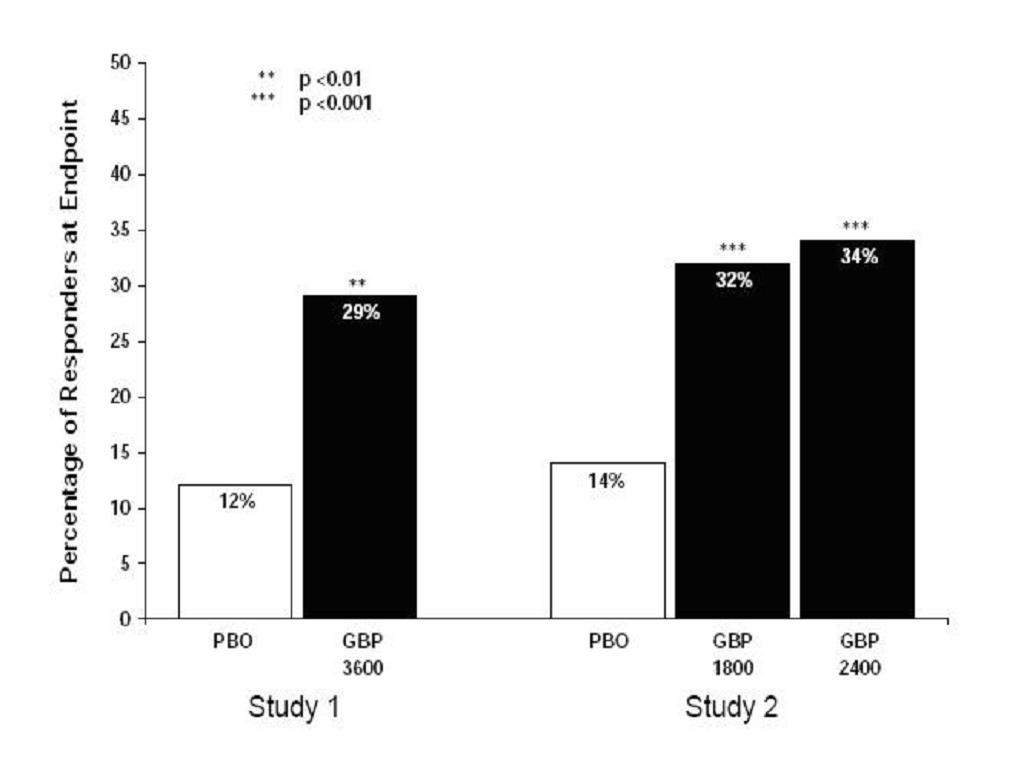
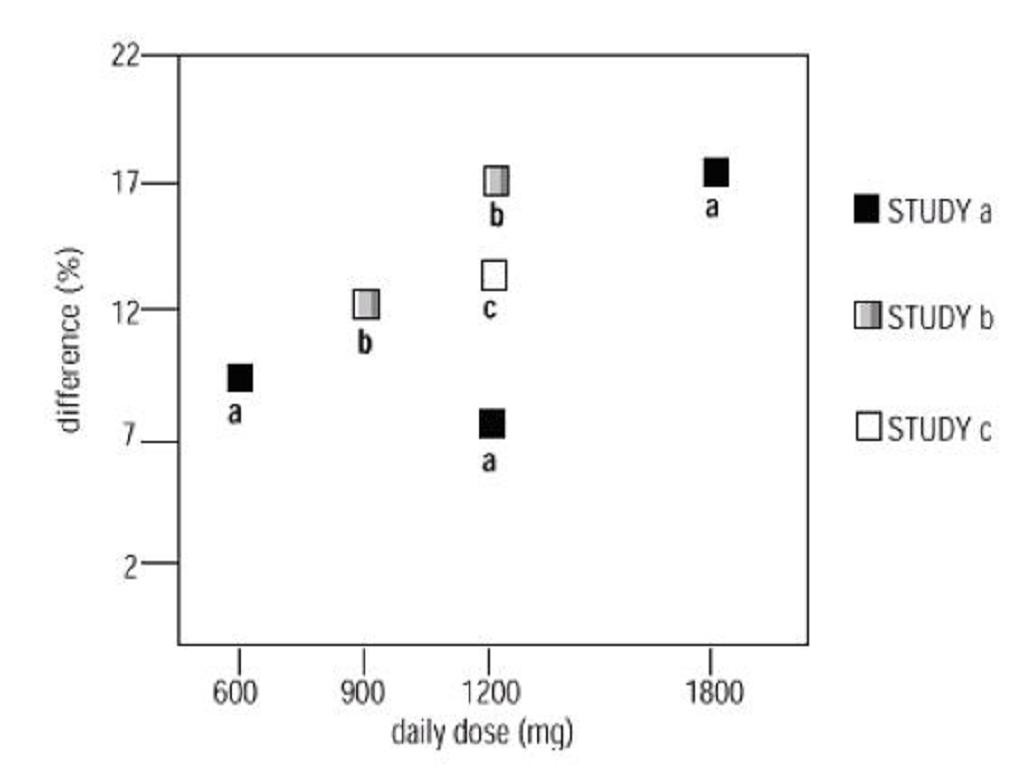
INDICATIONS & USAGE
Postherpetic NeuralgiaEpilepsy
GABAPENTIN CONTRAINDICATIONS
WARNINGS
PRECAUTIONS: Carcinogenesis, Mutagenesis, Impairment of Fertility
PRECAUTIONS
Information for PatientsDrug Interactions
PRECAUTIONS, Pregnancy
LABORATORY TESTS
DRUG INTERACTIONS
PRECAUTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGY, Clinical Studies
CLINICAL PHARMACOLOGY, Clinical Studies
GERIATRIC USE
CLINICAL PHARMACOLOGYADVERSE REACTIONSDOSAGE AND ADMINISTRATION
GABAPENTIN ADVERSE REACTIONS
WARNINGS, Neuropsychiatric Adverse Events
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
DOSAGE & ADMINISTRATION
CLINICAL PHARMACOLOGY, Pediatrics
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

GabapentinGabapentin CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!