G9 Whitening
TONYMOLY CO., LTD.
TONYMOLY CO., LTD.
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- G9 WHITENING INDICATIONS AND USAGE
- G9 WHITENING DOSAGE AND ADMINISTRATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
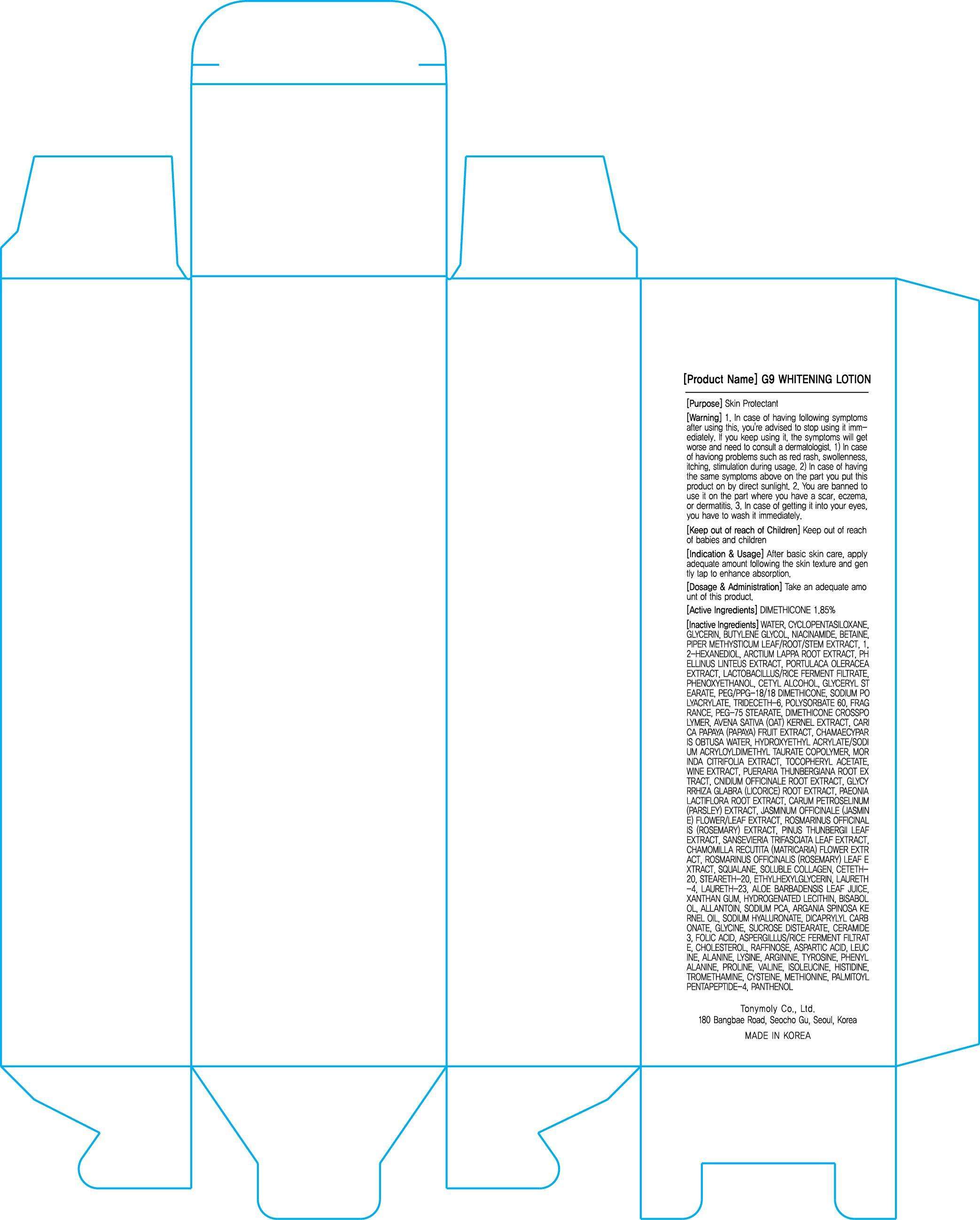
ACTIVE INGREDIENT
Active Ingredient: DIMETHICONE 1.85%
INACTIVE INGREDIENT
Inactive Ingredients:
WATER, CYCLOPENTASILOXANE, GLYCERIN, BUTYLENE GLYCOL, NIACINAMIDE, BETAINE, PIPER METHYSTICUM LEAF/ROOT/STEM EXTRACT, 1,2-HEXANEDIOL, ARCTIUM LAPPA ROOT EXTRACT, PHELLINUS LINTEUS EXTRACT, PORTULACA OLERACEA EXTRACT, LACTOBACILLUS/RICE FERMENT FILTRATE, PHENOXYETHANOL, CETYL ALCOHOL, GLYCERYL STEARATE, PEG/PPG-18/18 DIMETHICONE, SODIUM POLYACRYLATE, TRIDECETH-6, POLYSORBATE 60, FRAGRANCE, PEG-75 STEARATE, DIMETHICONE CROSSPOLYMER, AVENA SATIVA (OAT) KERNEL EXTRACT, CARICA PAPAYA (PAPAYA) FRUIT EXTRACT, CHAMAECYPARIS OBTUSA WATER, HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, MORINDA CITRIFOLIA EXTRACT, TOCOPHERYL ACETATE, WINE EXTRACT, PUERARIA THUNBERGIANA ROOT EXTRACT, CNIDIUM OFFICINALE ROOT EXTRACT, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, PAEONIA LACTIFLORA ROOT EXTRACT, CARUM PETROSELINUM (PARSLEY) EXTRACT, JASMINUM OFFICINALE (JASMINE) FLOWER/LEAF EXTRACT, ROSMARINUS OFFICINALIS (ROSEMARY) EXTRACT, PINUS THUNBERGII LEAF EXTRACT, SANSEVIERIA TRIFASCIATA LEAF EXTRACT, CHAMOMILLA RECUTITA (MATRICARIA) FLOWER EXTRACT, ROSMARINUS OFFICINALIS (ROSEMARY) LEAF EXTRACT, SQUALANE, SOLUBLE COLLAGEN, CETETH-20, STEARETH-20, ETHYLHEXYLGLYCERIN, LAURETH-4, LAURETH-23, ALOE BARBADENSIS LEAF JUICE, XANTHAN GUM, HYDROGENATED LECITHIN, BISABOLOL, ALLANTOIN, SODIUM PCA, ARGANIA SPINOSA KERNEL OIL, SODIUM HYALURONATE, DICAPRYLYL CARBONATE, GLYCINE, SUCROSE DISTEARATE, CERAMIDE 3, FOLIC ACID, ASPERGILLUS/RICE FERMENT FILTRATE, CHOLESTEROL, RAFFINOSE, ASPARTIC ACID, LEUCINE, ALANINE, LYSINE, ARGININE, TYROSINE, PHENYLALANINE, PROLINE, VALINE, ISOLEUCINE, HISTIDINE, TROMETHAMINE, CYSTEINE, METHIONINE, PALMITOYL PENTAPEPTIDE-4, PANTHENOL
PURPOSE
Purpose: skin Protectant
WARNINGS
Warnings:
1. In case of having following symptoms after using this, you're advised to stop using it immediately. If you keep using it, the symptoms will get worse and need to consult a dermatologist.
1) In case of haviong problems such as red rash, swollenness, itching, stimulation during usage.
2) In case of having the same symptoms above on the part you put this product on by direct sunlight.
2. You are banned to use it on the part where you have a scar, eczema, or dermatitis.
3. In case of getting it into your eyes, you have to wash it immediately.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children:
Keep out of reach of babies and children
G9 WHITENING INDICATIONS AND USAGE
INDICATIONS AND USAGE: After basic skin care, apply adequate amount following the skin texture and gently tap to enhance absorption.
G9 WHITENING DOSAGE AND ADMINISTRATION
DOSAGE AND ADMINISTRATION: Take an adequate amount of this product.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
G9 WhiteningDIMETHICONE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||