Fusion Beauty ColorCeuticals Acne Control Primer
Fusion Beauty ColorCeuticals Acne Control Primer
FULL PRESCRIBING INFORMATION
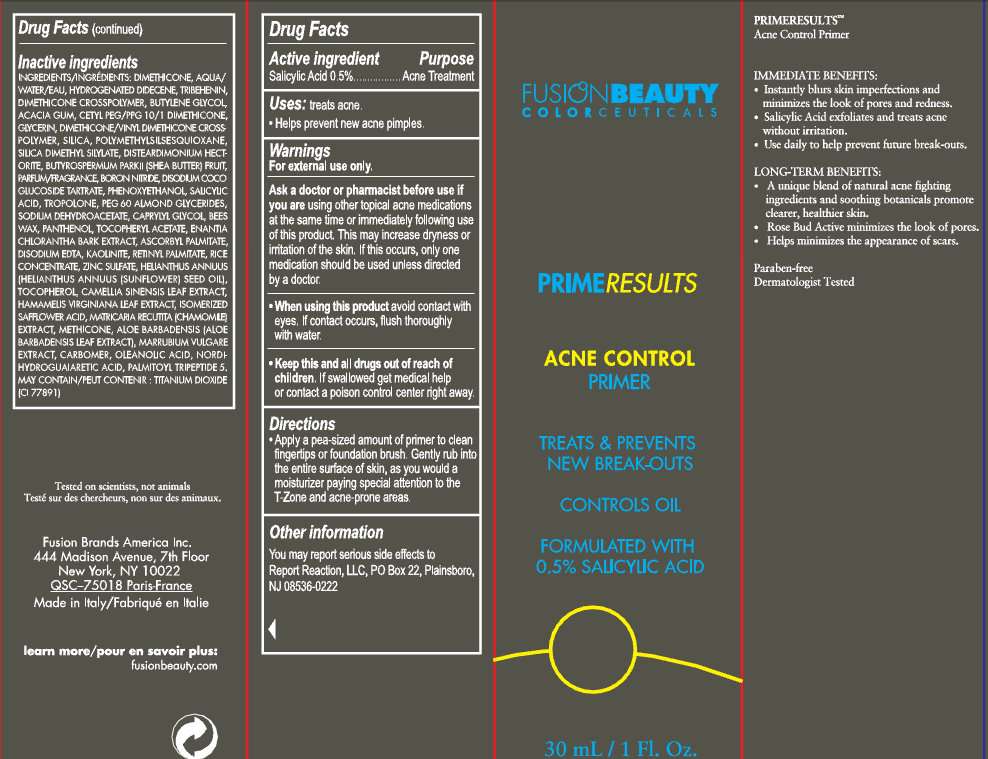
Active ingredient
Active ingredient Purpose
Salicylic Acid 0.5%.......................................................................Acne Treatment
Purpose
Uses: treats acne.
- Helps prevent new acne pimples.
Warnings
For external use only.
Ask a doctor or pharmacist before use if you are using other topical acne medications at the same time or immediately following use of this product. This may increase dryness or irritation of the skin. If the occurs, only one medication should be used unless directed by a doctor.
- When using this product avoid contact with eyes. If contact occurs, flush thoroughly with water.
- Keep this and all drugs out of reach of children. If swallowed get medical help or contact a poison control center right away.
Directions
- Apply a pea-sized amount of primer to clean fingertips or foundation brush. Gently rub into the entire surface of skin, as you would a moisturizer paying special attention to the T-Zone and acne-prone areas.
Other information
You may report serious side effects to Report Reaction, LLC, PO Box 22, Plainsboro, NJ 08536-0222
Inactive ingredients
INGREDIENTS: DIMETHICONE, AQUA/WATER/EAU, HYDROGENATED DIDECENE, TRIBEHENIN, DIMETHICONE CROSSPOLYMER, BUTYLENE GLYCOL, ACACIA GUM, CETYL PEG/PPG-10/1 DIMETHICONE, GLYCERIN, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, SILICA, POLYMETHYLSILSESQUIOXANE, SILICA DIMETHYL SILYLATE, DISTEARDIMONIUM HECTORITE, BUTYROSPERMUM PARKII (SHEA BUTTER) FRUIT, PARFUM/FRAGRANCE, BORON NITRIDE, DISODIUM COCOGLUCOSIDE TARTRATE, PHENOXYETHANOL, SALICYLIC ACID, TROPOLONE, PEG-60 ALMOND GLYCERIDES, SODIUM DEHYDROACETATE, CAPRYLYL GLYCOL, BEES WAX, PANTHENOL, TOCOPHERYL ACETATE, ENANTIA CHLORANTHA BARK EXTRACT, ASCORBYL PALMITATE, DISODIUM EDTA, KAOLINITE, RETINYL PALMITATE, RICE CONCENTRATE, ZINC SULFATE, HELIANTHUS ANNUUS (HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL), TOCOPHEROL, CAMELLIA SINENSIS LEAF EXTRACT, HAMAMELIS VIRGINIANA LEAF EXTRACT, ISOMERIZED SAFFLOWER ACID, MATRICARIA RECUTITA (CHAMOMILE) EXTRACT, METHICONE, ALOE BARBADENSIS (ALOEBARBADENSIS LEAF EXTRACT), MARRUBIUM VULGARE EXTRACT, CARBOMER, OLEANOLIC ACID, NORDIHYDROGUAIARETIC ACID, PALMITOYL TRIPEPTIDE-5.
MAY CONTAIN: TITANIUM DIOXIDE (CI 77891)
Tested on scientists, not animals
Fusion Brands America Inc.
444 Madison Avenue, 7th Floor
New York, NY 10022
QSC-75018 Paris-France
Made in italy
learn more: fusionbeauty.com
PRIMERESULTS
Acne Control Primer
QSC-75018 Paris-France

FUSIONBEAUTY
C O L O R C E U T I C A L S
PRIMERESULTS
ACNE CONTROL
PRIMER
BASE
ANTI-ACNE
acne control
Fusion Beauty ColorCeuticals Acne Control PrimerSALICYLIC ACID LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||