fomepizole
Rx only Sterile Caution: Must be diluted prior to use.
FULL PRESCRIBING INFORMATION: CONTENTS*
- FOMEPIZOLE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- FOMEPIZOLE CONTRAINDICATIONS
- PRECAUTIONS
- FOMEPIZOLE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
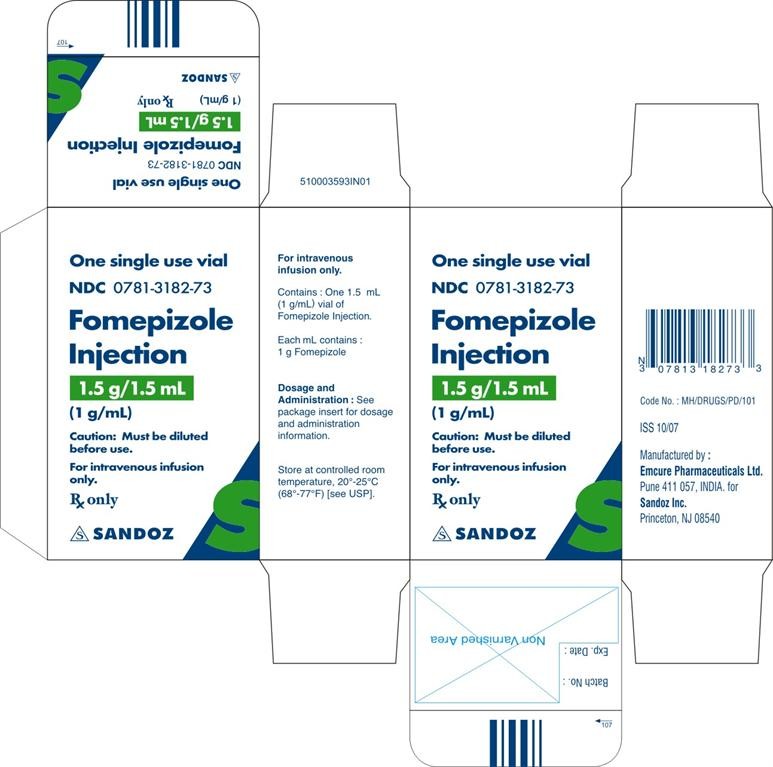
- PACKAGE CARTON PRINCIPAL DISPLAY PANEL
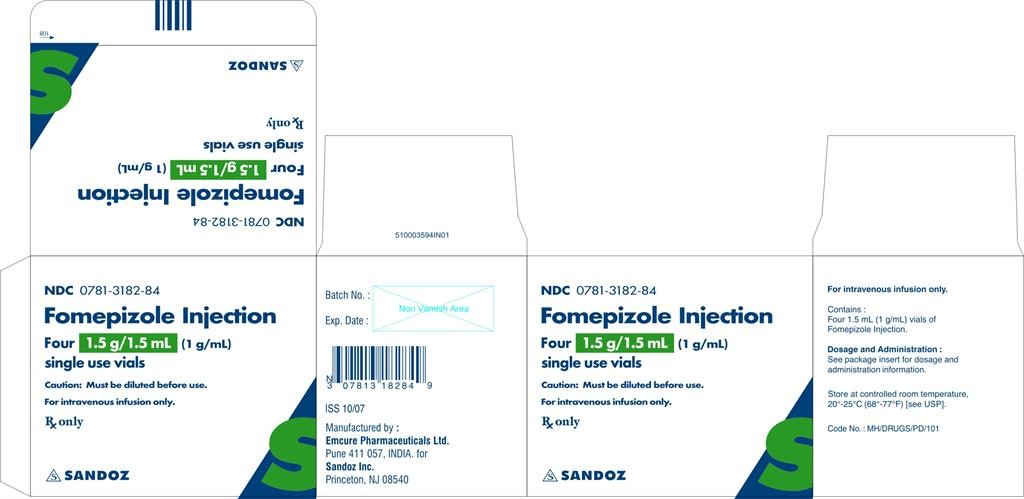
- PACKAGE OUTER CARTON PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
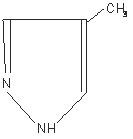
FOMEPIZOLE DESCRIPTION
462
CLINICAL PHARMACOLOGY
Mechanism of Action
PRECAUTIONSDrug Interactions
Pharmacokinetics
Distribution
Metabolism
Excretion
Special Populations
Geriatric
Pediatric
Gender
Renal Insufficiency
Hepatic Insufficiency
Clinical Studies
DOSAGE AND ADMINISTRATION
INDICATIONS & USAGE
DOSAGE AND ADMINISTRATION
FOMEPIZOLE CONTRAINDICATIONS
PRECAUTIONS
General
ADVERSE REACTIONS
Laboratory Tests
DOSAGE AND ADMINISTRATION
Drug Interactions
Carcinogenesis & Mutagenesis & Impairment Of Fertility
Pregnancy
Pregnancy Category C : Animal reproduction studies have not been conducted with fomepizole. It is also not known whether fomepizole can cause fetal harm when administered to pregnant women or can affect reproduction capacity. Fomepizole should be given to pregnant women only if clearly needed.
Nursing Mothers
Pediatric Use
Geriatric Use
FOMEPIZOLE ADVERSE REACTIONS
Body as a Whole
Cardiovascular
Gastrointestinal
Hemic/Lymphatic
Nervous
Respiratory
Skin/Appendages
Special Senses
Urogenital
OVERDOSAGE
DOSAGE & ADMINISTRATION
Treatment Guidelines
Treatment with fomepizole
Hemodialysis
Discontinuation of fomepizole Treatment
Dosing of fomepizole
Dosage with Renal Dialysis
Fomepizole Dosing in Patients Requiring Hemodialysis
| DOSE AT THE BEGINNING OF HEMODIALYSIS | |
| If <6 hours since last fomepizole dose | If ≥ 6 hours since last fomepizole dose |
| Do not administer dose | Administer next scheduled dose |
| DOSING DURING HEMODIALYSIS |
| Dose every 4 hours |
| DOSING AT THE TIME HEMODIALYSIS IS COMPLETED | |
|
Time between last dose and the end of hemodialysis |
|
| <1 hour | Do not administer dose at the end of hemodialysis |
| 1-3 hours | Administer 1/2 of next scheduled dose |
| >3 hours | Administer next scheduled dose |
| MAINTENANCE DOSING OFF HEMODIALYSIS |
| Give next scheduled dose 12 hours from last dose administered |
Administration
Stability : Fomepizole diluted in 0.9% sodium chloride injection or dextrose 5% injection remains stable and sterile for at least 24 hours when stored refrigerated or at room temperature. Fomepizole does not contain preservatives. Therefore, maintain sterile conditions, and after dilution do not use beyond 24 hours.
Solutions showing haziness, particulate matter, precipitate, discoloration, or leakage should not be used.
HOW SUPPLIED
Manufactured By
Emcure Pharmaceuticals Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

PACKAGE CARTON PRINCIPAL DISPLAY PANEL
PACKAGE OUTER CARTON PRINCIPAL DISPLAY PANEL
fomepizolefomepizole INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!