Fluphenazine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- FLUPHENAZINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- FLUPHENAZINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PREGNANCY
- FLUPHENAZINE HYDROCHLORIDE ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
FLUPHENAZINE HYDROCHLORIDE DESCRIPTION
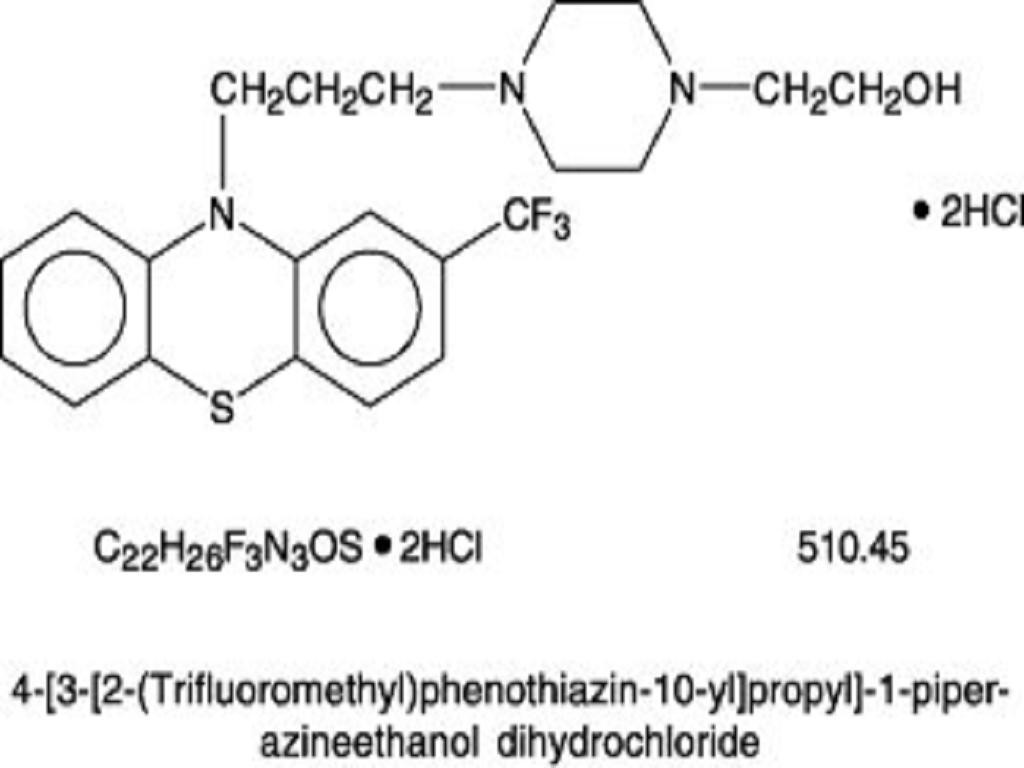
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
FLUPHENAZINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related PsychosisBOXED WARNING
Tardive Dyskinesia
PRECAUTIONS: Information for PatientsADVERSE REACTIONS: Tardive Dyskinesia
Neuroleptic Malignant Syndrome (NMS)
PRECAUTIONS
Leukopenia, Neutropenia and AgranulocytosisGeneral
Information for Patients
Abrupt Withdrawal
PREGNANCY
Non-teratogenic EffectsFLUPHENAZINE HYDROCHLORIDE ADVERSE REACTIONS
Central Nervous SystemExtrapyramidal Symptoms
Dystonia
Class Effect
Tardive Dyskinesia
WARNINGS
Other CNS Effects
WARNINGS: Neuroleptic Malignant Syndrome (NMS)
Drowsiness or lethargy, if they occur, may necessitate a reduction in dosage; the induction of a catatonic-like state has been known to occur with dosages of fluphenazine far in excess of the recommended amounts. As with other phenothiazine compounds, reactivation or aggravation of psychotic processes may be encountered.
Phenothiazine derivatives have been known to cause, in some patients, restlessness, excitement, or bizarre dreams.
Autonomic Nervous System
Hypertension and fluctuations in blood pressure have been reported with fluphenazine hydrochloride.
Hypotension has rarely presented a problem with fluphenazine. However, patients with pheochromocytoma, cerebral vascular or renal insufficiency, or a severe cardiac reserve deficiency such as mitral insufficiency appear to be particularly prone to hypotensive reactions with phenothiazine compounds, and should therefore be observed closely when the drug is administered. If severe hypotension should occur, supportive measures including the use of intravenous vasopressor drugs should be instituted immediately. Norepinephrine bitartrate injection is the most suitable drug for this purpose; epinephrine should not be used since phenothiazine derivatives have been found to reverse its action, resulting in a further lowering of blood pressure.
Autonomic reactions including nausea and loss of appetite, salivation, polyuria, perspiration, dry mouth, headache, and constipation may occur. Autonomic effects can usually be controlled by reducing or temporarily discontinuing dosage.
In some patients, phenothiazine derivatives have caused blurred vision, glaucoma, bladder paralysis, fecal impaction, paralytic ileus, tachycardia, or nasal congestion.
Metabolic and Endocrine
Weight change, peripheral edema, abnormal lactation, gynecomastia, menstrual irregularities, false results on pregnancy tests, impotency in men and increased libido in women have all been known to occur in some patients on phenothiazine therapy.
Allergic Reactions
Skin disorders such as itching, erythema, urticaria, seborrhea, photosensitivity, eczema and even exfoliative dermatitis have been reported with phenothiazine derivatives. The possibility of anaphylactoid reactions occurring in some patients should be borne in mind.
Hematologic
Routine blood counts are advisable during therapy since blood dyscrasias including leukopenia, agranulocytosis, thrombocytopenic or non-thrombocytopenic purpura, eosinophilia, and pancytopenia have been observed with phenothiazine derivatives. Furthermore, if any soreness of the mouth, gums, or throat, or any symptoms of upper respiratory infection occur and confirmatory leukocyte count indicates cellular depression, therapy should be discontinued and other appropriate measures instituted immediately.
Hepatic
Liver damage as manifested by cholestatic jaundice may be encountered, particularly during the first months of therapy; treatment should be discontinued if this occurs. An increase in cephalin flocculation, sometimes accompanied by alterations in other liver function tests, has been reported in patients receiving fluphenazine hydrochloride who have had no clinical evidence of liver damage.
Others
Although this is not a general feature of fluphenazine, potentiation of central nervous system depressants (opiates, analgesics, antihistamines, barbiturates, alcohol) may occur.
The following adverse reactions have also occurred with phenothiazine derivatives: systemic lupus erythematosus-like syndrome, hypotension severe enough to cause fatal cardiac arrest, altered electrocardiographic and electroencephalographic tracings, altered cerebrospinal fluid proteins, cerebral edema, asthma, laryngeal edema, and angioneurotic edema; with long-term useskin pigmentation, and lenticular and corneal opacities.
DOSAGE & ADMINISTRATION
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Fluphenazine HydrochlorideFluphenazine Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!