Fluoxetine
Lake Erie Medical DBA Quality Care Products LLC
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use fluoxetine safely and effectively. See full prescribing information for fluoxetine capsules. Fluoxetine Capsules, USP for Oral Use Initial U.S. Approval: 1987 RECENT MAJOR CHANGES(1.5)(2.5)(5.2)BOXED WARNING WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS See full prescribing information for complete boxed warning. Increased risk of suicidal thinking and behavior in children, adolescents, and young adults taking antidepressants for Major Depressive Disorder (MDD) and other psychiatric disorders (5.1). When using fluoxetine and olanzapine in combination, also refer to Boxed Warning section of the package insert for Symbyax. INDICATIONS AND USAGE Acute and maintenance treatment of Major Depressive Disorder (MDD) in adult and pediatric patients aged 8 to 18 years (1.1) Acute and maintenance treatment of Obsessive Compulsive Disorder (OCD) in adult and pediatric patients aged 7 to 17 years (1.2) Acute and maintenance treatment of Bulimia Nervosa in adult patients (1.3) Acute treatment of Panic Disorder, with or without agoraphobia, in adult patients (1.4) Fluoxetine capsules and olanzapine in combination for: Acute treatment of Depressive Episodes Associated with Bipolar I Disorder in adults (1.5) DOSAGE AND ADMINISTRATION Indication Adult Pediatric MDD (2.1) 20 mg/day in am (initial dose) 10 to 20 mg/day (initial dose) OCD (2.2) 20 mg/day in am (initial dose) 10 mg/day (initial dose) Bulimia Nervosa (2.3) 60 mg/day in am - Panic Disorder (2.4) 10 mg/day (initial dose) - Depressive Episodes Associated with Bipolar I Disorder (2.5) Oral in combination with olanzapine: 5 mg of oral olanzapine and 20 mg of fluoxetine once daily (initial dose) - Consider tapering the dose of fluoxetine for pregnant women during the third trimester (2.7) A lower or less frequent dosage should be used in patients with hepatic impairment, the elderly, and for patients with concurrent disease or on multiple concomitant medications (2.7) Fluoxetine capsules and olanzapine in combination: Dosage adjustments, if indicated, should be made with the individual components according to efficacy and tolerability (2.5) Fluoxetine monotherapy is not indicated for the treatment of Depressive Episodes associated with Bipolar I Disorder (2.5) Safety of the coadministration of doses above 18 mg olanzapine with 75 mg fluoxetine has not been evaluated (2.5) DOSAGE FORMS AND STRENGTHS Capsules: 10 mg, 20 mg, and 40 mg (3) CONTRAINDICATIONS Do not use with an MAOI or within 14 days of discontinuing an MAOI due to risk of drug interaction. At least 5 weeks should be allowed after stopping fluoxetine capsules before treatment with an MAOI (4, 7.1) Do not use with pimozide due to risk of drug interaction or QTc prolongation (4, 7.9) Do not use with thioridazine due to QTc interval prolongation or potential for elevated thioridazine plasma levels. Do not use thioridazine within 5 weeks of discontinuing fluoxetine capsules (4, 7.9) When using fluoxetine capsules and olanzapine in combination, also refer to the Contraindications section of the package insert for Symbyax (4) WARNINGS AND PRECAUTIONS Clinical Worsening and Suicide Risk: Monitor for clinical worsening and suicidal thinking and behavior (5.1) Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions: Have been reported with fluoxetine. Discontinue fluoxetine and initiate supportive treatment (5.2) Allergic Reactions and Rash: Discontinue upon appearance of rash or allergic phenomena (5.3) Activation of Mania/Hypomania: Screen for Bipolar Disorder and monitor for mania/hypomania (5.4) Seizures: Use cautiously in patients with a history of seizures or with conditions that potentially lower the seizure threshold (5.5) Altered Appetite and Weight: Significant weight loss has occurred (5.6) Abnormal Bleeding: May increase the risk of bleeding. Use with NSAIDs, aspirin, warfarin, or drugs that affect coagulation may potentiate the risk of gastrointestinal or other bleeding (5.7) Hyponatremia: Has been reported with fluoxetine in association with syndrome of inappropriate antidiuretic hormone (SIADH) (5.8) Anxiety and Insomnia: May occur (5.9) Potential for Cognitive and Motor Impairment: Has potential to impair judgment, thinking, and motor skills. Use caution when operating machinery (5.11) Long Half-Life: Changes in dose will not be fully reflected in plasma for several weeks (5.12) Fluoxetine and Olanzapine in Combination: When using fluoxetine and olanzapine in combination, also refer to the Warnings and Precautions section of the package insert for Symbyax (5.14) Side Effects6.1To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS Monoamine Oxidase Inhibitors (MAOI): Fluoxetine is contraindicated for use with MAOI’s, or within 14 days of discontinuing an MAOI due to risk of drug interaction. At least 5 weeks should be allowed after stopping fluoxetine before starting treatment with an MAOI (4, 7.1) Pimozide: Fluoxetine is contraindicated for use with pimozide due to risk of drug interaction or QTc prolongation (4, 7.9) Thioridazine: Fluoxetine is contraindicated for use with thioridazine due to QTc interval prolongation or potential for elevated thioridazine plasma levels. Do not use thioridazine within 5 weeks of discontinuing fluoxetine (4, 7.9) Drugs Metabolized by CYP2D6: Fluoxetine is a potent inhibitor of CYP2D6 enzyme pathway (7.9) Tricyclic Antidepressants (TCAs): Monitor TCA levels during coadministration with fluoxetine or when fluoxetine has been recently discontinued (7.9) CNS Acting Drugs: Caution should be used when taken in combination with other centrally acting drugs (7.2) Benzodiazepines: Diazepam - increased t½, alprazolam – further psychomotor performance decrement due to increased levels (7.9) Antipsycotics: Potential for elevation of haloperidol and clozapine levels (7.9) Anticonvulsants: Potential for elevated phenytoin and carbamazepine levels and clinical anticonvulsant toxicity (7.9) Serotonergic Drugs: Potential for Serotonin Syndrome (5.2, 7.3) Triptans: There have been rare postmarketing reports of Serotonin Syndrome with use of an SSRI and a triptan (5.2, 7.4) Tryptophan: Concomitant use with tryptophan is not recommended (5.2, 7.5) Drugs that Interfere with Hemostasis (e.g., NSAIDs, Aspirin, Warfarin): May potentiate the risk of bleeding (7.6) Drugs Tightly Bound to Plasma Proteins: May cause a shift in plasma concentrations (7.8, 7.9) Olanzapine: When used in combination with fluoxetine, also refer to the Drug Interactions section of the package insert for Symbyax (7.9) USE IN SPECIFIC POPULATIONS Pregnancy: Fluoxetine should be used during pregnancy only if the potential benefit justifies the potential risks to the fetus (8.1) Nursing Mothers: Breast feeding is not recommended (8.3) Pediatric Use: Safety and effectiveness of fluoxetine and olanzapine in combination have not been established in patients less than 18 years of age (8.4) Hepatic Impairment: Lower or less frequent dosing may be appropriate in patients with cirrhosis (8.6)
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS
- 1 FLUOXETINE INDICATIONS AND USAGE
- 2 FLUOXETINE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 FLUOXETINE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Clinical Worsening and Suicide Risk
- 5.2 Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
- 5.3 Allergic Reactions and Rash
- 5.4 Screening Patients for Bipolar Disorder and Monitoring for Mania/Hypomania
- 5.5 Seizures
- 5.6 Altered Appetite and Weight
- 5.7 Abnormal Bleeding
- 5.8 Hyponatremia
- 5.9 Anxiety and Insomnia
- 5.10 Use in Patients with Concomitant Illness
- 5.11 Potential for Cognitive and Motor Impairment
- 5.12 Long Elimination Half-Life
- 5.13 Discontinuation of Treatment
- 5.14 Fluoxetine and Olanzapine in Combination
- 6 FLUOXETINE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 7.1 Monoamine Oxidase Inhibitors
- 7.2 CNS Acting Drugs
- 7.3 Serotonergic Drugs
- 7.4 Triptans
- 7.5 Tryptophan
- 7.6 Drugs that Interfere with Hemostasis (e.g., NSAIDs, Aspirin, Warfarin)
- 7.7 Electroconvulsive Therapy (ECT)
- 7.8 Potential for Other Drugs to affect Fluoxetine
- 7.9 Potential for Fluoxetine to affect Other Drugs
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 FLUOXETINE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- 17.1 General Information
- 17.2 Clinical Worsening and Suicide Risk
- 17.3 Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
- 17.4 Allergic Reactions and Rash
- 17.5 Abnormal Bleeding
- 17.6 Hyponatremia
- 17.7 Potential for Cognitive and Motor Impairment
- 17.8 Use of Concomitant Medications
- 17.9 Discontinuation of Treatment
- 17.10 Use in Specific Populations
- Medication Guide
FULL PRESCRIBING INFORMATION
WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of Major Depressive Disorder (MDD) and other psychiatric disorders. Anyone considering the use of fluoxetine or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Fluoxetine is approved for use in pediatric patients with MDD and Obsessive Compulsive Disorder (OCD) [see Warnings and Precautions (5.1) and Use in Specific Populations ( 8.4) ].
When using fluoxetine and olanzapine in combination, also refer to Boxed Warning section of the package insert for Symbyax.
1 INDICATIONS AND USAGE
1.1 Major Depressive Disorder
[see Clinical Studies (14.1)].
[see Dosage and Administration (2.1)]
1.2 Obsessive Compulsive Disorder
[see Clinical Studies (14.2)].
[see Dosage and Administration (2.2)].
1.3 Bulimia Nervosa
[see Clinical Studies (14.3)].
[see Dosage and Administration (2.3)]
1.4 Panic Disorder
[see Clinical Studies (14.4) ]
[see Dosage and Administration (2.4)].
1.5 Fluoxetine Capsules and Olanzapine in Combination: Depressive Episodes Associated with Bipolar I Disorder
When using fluoxetine capsules and olanzapine in combination, also refer to the Clinical Studies section of the package insert for Symbyax®
in
2 DOSAGE AND ADMINISTRATION
2.1 Major Depressive Disorder
Initial Treatment
Adult
Pediatric (children and adolescents) [see Clinical Studies (14.1)]
All patients
Maintenance/Continuation/Extended Treatment
Daily Dosing [see Clinical Studies (14.1)]
Switching Patients to a Tricyclic Antidepressant (TCA) [see Drug Interactions (7.9) ]
Switching Patients to or from a Monoamine Oxidase Inhibitor (MAOI) [see Contraindications (4) and Drug Interactions (7.1)]
2.2 Obsessive Compulsive Disorder
Initial Treatment
Adult [see Clinical Studies (14.2)]
Pediatric (children and adolescents) [see Clinical Studies (14.2)]
Maintenance/Continuation Treatment
2.3 Bulimia Nervosa
Initial Treatment [see Clinical Studies (14.3)]
Maintenance/Continuation Treatment [see Clinical Studies (14.3)]
2.4 Panic Disorder
Initial Treatment [see Clinical Studies (14.4)]
Maintenance/Continuation Treatment
2.5 Fluoxetine Capsules and Olanzapine in Combination: Depressive Episodes Associated with Bipolar I Disorder
When using fluoxetine capsules and olanzapine in combination, also refer to the Clinical Studies section of the package insert for Symbyax
in
in
| For Symbyax (mg/day) | Use in Combination | |
|---|---|---|
| Olanzapine (mg/day) |
Fluoxetine (mg/day) |
|
|
1 Symbyax (olanzapine/fluoxetine hydrochloride) is a fixed-dose combination of fluoxetine and olanzapine. |
||
| 3 mg olanzapine/25 mg fluoxetine |
2.5 |
20 |
| 6 mg olanzapine/25 mg fluoxetine |
5 |
20 |
| 12 mg olanzapine/25 mg fluoxetine |
10+2.5 |
20 |
| 6 mg olanzapine/50 mg fluoxetine |
5 |
40+10 |
| 12 mg olanzapine/50 mg fluoxetine |
10+2.5 |
40+10 |
The
with
the
2.7 Dosing in Specific Populations
Treatment of pregnant Women During the Third Trimester [see Use in Specific Populations (8.1)].
Geriatrics [see Use in Specific Populations (8.5)].
Hepatic Impairment [see Clinical Pharmacology (12.4) and Use in Specific Populations (8.6)].
Concomitant Illness [see Clinical Pharmacology (12.4) and Warnings and Precautions (5.10)].
Fluoxetine Capsules and Olanzapine in Combination [see Warnings and Precautions (5.14) and Drug Interactions (7.9)]
2.8 Discontinuation of Treatment
[see Warnings and Precautions (5.13)].
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
When using fluoxetine capsules and olanzapine in combination, also refer to the Contraindications section of the package insert for Symbyax.
- Monoamine Oxidase Inhibitors [see Drug Interactions (7.1)]
- Pimozide [see Drug Interactions (7.9)]
- Thioridazine [see Drug Interactions (7.9)]
5 WARNINGS AND PRECAUTIONS
When using Fluoxetine and olanzapine in combination, also refer to the Warnings and Precautions section of the package insert for Symbyax.
5.1 Clinical Worsening and Suicide Risk
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated |
|---|---|
| Increases Compared to Placebo |
|
| <18 |
14 additional cases |
| 18-24 |
5 additional cases |
| Decreases Compared to Placebo |
|
| 25-64 |
1 fewer case |
| ≥65 |
6 fewer cases |
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
[see Warnings and Precautions (5.13)]
Families and caregivers of patients being treated with antidepressants for Major Depressive Disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.
5.2 Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
SSRIs alone, including fluoxetine treatment, but particularly with concomitant use of serotonergic drugs (including triptans) with drugs which impair metabolism of
The concomitant use of [see Contraindications (4) and Drug Interactions (7.1)]
If concomitant treatment of fluoxetine [see Drug Interactions (7.4)]
The concomitant use of fluoxetine with serotonin [see Drug Interactions (7.3) ]
Treatment with fluoxetine and any concomitant serotonergic
5.3 Allergic Reactions and Rash
5.4 Screening Patients for Bipolar Disorder and Monitoring for Mania/Hypomania
[see Warnings and Precautions section of the package insert for Symbyax]
[see Use in Specific Populations (8.4)]
[see Use in Specific Populations (8.4)]
5.5 Seizures
5.6 Altered Appetite and Weight
[see Use in Specific Populations (8.4)]
[see Use in Specific Populations (8.4) ]
5.7 Abnormal Bleeding
[see Drug Interactions (7.6)]
5.8 Hyponatremia
[see Use in Specific Populations (8.5)]
5.9 Anxiety and Insomnia
[see Table 5]
5.10 Use in Patients with Concomitant Illness
Cardiovascular
Glycemic Control
5.11 Potential for Cognitive and Motor Impairment
5.12 Long Elimination Half-Life
[see Clinical Pharmacology (12.3)]
5.13 Discontinuation of Treatment
5.14 Fluoxetine and Olanzapine in Combination
When using fluoxetine and olanzapine in combination, also refer to the Warnings and Precautions section of the package insert for Symbyax.
6 ADVERSE REACTIONS
When using fluoxetine and olanzapine in combination, also refer to the Adverse Reactions section of the package insert for Symbyax.
6.1 Clinical Trials Experience
Incidence in Major Depressive Disorder, OCD, bulimia, and Panic Disorder placebo-controlled clinical trials (excluding data from extensions of trials)
| Percentage of Patients Reporting Event | ||||||||
|---|---|---|---|---|---|---|---|---|
| Major Depressive Disorder |
OCD | Bulimia | Panic Disorder | |||||
|
1 Incidence less than 1%. 2 Includes U.S. data for Major Depressive Disorder, OCD, Bulimia, and Panic Disorder clinical trials, plus non-U.S. data for Panic Disorder clinical trials. 3 Denominator used was for males only (N=690 fluoxetine Major Depressive Disorder; N=410 placebo Major Depressive Disorder; N=116 fluoxetine OCD; N=43 placebo OCD; N=14 fluoxetine bulimia; N=1 placebo bulimia; N=162 fluoxetine panic; N=121 placebo panic). |
||||||||
|
Body System/
Adverse Reaction
|
Fluoxetine
(N=1728) |
Placebo
(N=975) |
Fluoxetine
(N=266) |
Placebo
(N=89) |
Fluoxetine
(N=450) |
Placebo
(N=267) |
Fluoxetine
(N=425) |
Placebo
(N=342) |
|
Body as a Whole
|
||||||||
| Asthenia |
9 |
5 |
15 |
11 |
21 |
9 |
7 |
7 |
| Flu syndrome |
3 |
4 |
10 |
7 |
8 |
3 |
5 |
5 |
|
Cardiovascular
System
|
||||||||
| Vasodilatation |
3 |
2 |
5 |
-- |
2 |
1 |
1 |
-- |
|
Digestive System
|
||||||||
| Nausea |
21 |
9 |
26 |
13 |
29 |
11 |
12 |
7 |
| Diarrhea |
12 |
8 |
18 |
13 |
8 |
6 |
9 |
4 |
| Anorexia |
11 |
2 |
17 |
10 |
8 |
4 |
4 |
1 |
| Dry mouth |
10 |
7 |
12 |
3 |
9 |
6 |
4 |
4 |
| Dyspepsia |
7 |
5 |
10 |
4 |
10 |
6 |
6 |
2 |
|
Nervous System
|
||||||||
| Insomnia |
16 |
9 |
28 |
22 |
33 |
13 |
10 |
7 |
| Anxiety |
12 |
7 |
14 |
7 |
15 |
9 |
6 |
2 |
| Nervousness |
14 |
9 |
14 |
15 |
11 |
5 |
8 |
6 |
| Somnolence |
13 |
6 |
17 |
7 |
13 |
5 |
5 |
2 |
| Tremor |
10 |
3 |
9 |
1 |
13 |
1 |
3 |
1 |
| Libido decreased |
3 |
-- |
11 |
2 |
5 |
1 |
1 |
2 |
| Abnormal dreams |
1 |
1 |
5 |
2 |
5 |
3 |
1 |
1 |
|
Respiratory System
|
||||||||
| Pharyngitis |
3 |
3 |
11 |
9 |
10 |
5 |
3 |
3 |
| Sinusitis |
1 |
4 |
5 |
2 |
6 |
4 |
2 |
3 |
| Yawn |
-- |
-- |
7 |
-- |
11 |
-- |
1 |
-- |
|
Skin and Appendages
|
||||||||
| Sweating |
8 |
3 |
7 |
-- |
8 |
3 |
2 |
2 |
| Rash |
4 |
3 |
6 |
3 |
4 |
4 |
2 |
2 |
|
Urogenital System
|
||||||||
| Impotence3
|
2 |
-- |
-- |
-- |
7 |
-- |
1 |
-- |
| Abnormal ejaculation3
|
-- |
-- |
7 |
-- |
7 |
-- |
2 |
1 |
| Percentage of Patients Reporting Event | ||
|---|---|---|
| Major Depressive Disorder, OCD, Bulimia, and Panic Disorder Combined |
||
|
1 Incidence less than 1%. 2 Includes U.S. data for Major Depressive Disorder, OCD, bulimia, and Panic Disorder clinical trials, plus non-U.S. data for Panic Disorder clinical trials. |
||
|
Body System/Adverse Reaction
|
Fluoxetine
(N=2869) |
Placebo
(N=1673) |
|
Body as a Whole
|
||
| Headache |
21 |
19 |
| Asthenia |
11 |
6 |
| Flu syndrome |
5 |
4 |
| Fever |
2 |
1 |
|
Cardiovascular System
|
||
| Vasodilatation |
2 |
1 |
|
Digestive System
|
||
| Nausea |
22 |
9 |
| Diarrhea |
11 |
7 |
| Anorexia |
10 |
3 |
| Dry mouth |
9 |
6 |
| Dyspepsia |
8 |
4 |
| Constipation |
5 |
4 |
| Flatulence |
3 |
2 |
| Vomiting |
3 |
2 |
|
Metabolic and Nutritional Disorders
|
||
| Weight loss |
2 |
1 |
|
Nervous System
|
||
| Insomnia |
19 |
10 |
| Nervousness |
13 |
8 |
| Anxiety |
12 |
6 |
| Somnolence |
12 |
5 |
| Dizziness |
9 |
6 |
| Tremor |
9 |
2 |
| Libido decreased |
4 |
1 |
| Thinking abnormal |
2 |
1 |
|
Respiratory System
|
||
| Yawn |
3 |
-- |
|
Skin and Appendages
|
||
| Sweating |
7 |
3 |
| Rash |
4 |
3 |
| Pruritus |
3 |
2 |
|
Special Senses
|
||
| Abnormal vision |
2 |
1 |
|
1 Includes U.S. Major Depressive Disorder, OCD, bulimia, and Panic Disorder clinical trials, plus non-U.S. Panic Disorder clinical trials. |
||||
|
Major Depressive Disorder, OCD, Bulimia, and Panic Disorder Combined
(N=1533) |
Major Depressive Disorder
(N=392) |
OCD
(N=266) |
Bulimia
(N=450) |
Panic Disorder
(N=425) |
| Anxiety (1%)
|
--
|
Anxiety (2%)
|
--
|
Anxiety (2%)
|
| --
|
--
|
--
|
Insomnia (2%)
|
--
|
| --
|
Nervousness (1%)
|
--
|
--
|
Nervousness (1%)
|
| --
|
--
|
Rash (1%)
|
--
|
--
|
Other adverse reactions in pediatric patients (children and adolescents)
Male and female sexual dysfunction with SSRIs
6.2 Other Reactions
Body as a Whole Frequent: Infrequent: Rare:
Cardiovascular System Frequent: Infrequent:
Digestive System Infrequent: Rare:
Hemic and Lymphatic System Infrequent: Rare:
Nervous System Frequent: Infrequent: Rare:
Respiratory System Rare:
Skin and Appendages Rare:
Special Senses Frequent: Infrequent:
6.3 Postmarketing Experience
1111111
1 These terms represent serious adverse events, but do not meet the definition for adverse drug reactions. They are included here because of their seriousness.
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase Inhibitors
[see Contraindications (4)][see Clinical Pharmacology (12.3)]
7.2 CNS Acting Drugs
[see Clinical Pharmacology (12.3)]
7.3 Serotonergic Drugs
[see Warnings and Precautions (5.2)][see Drug Interactions (7.4), (7.5)]
7.4 Triptans
[see Warnings and Precautions (5.2) and Drug Interactions (7.3)]
7.5 Tryptophan
[see Warnings and Precautions (5.2) and Drug Interactions (7.3)]
7.6 Drugs that Interfere with Hemostasis (e.g., NSAIDs, Aspirin, Warfarin)
[see Warnings and Precautions (5.7)]
7.7 Electroconvulsive Therapy (ECT)
7.8 Potential for Other Drugs to affect Fluoxetine
Drugs Tightly Bound to Plasma Proteins [see Clinical Pharmacology (12.3)]
7.9 Potential for Fluoxetine to affect Other Drugs
Pimozide —cc[see Contraindications (4)]
Thioridazine —[see Contraindications (4)]
max
c
Drugs Metabolized by CYP2D6 —[see Contraindications (4)]
Tricyclic Antidepressants (TCAs) — [see Clinical Pharmacology (12.3)]
Benzodiazapines — [see Clinical Pharmacology (12.3)]
Antipsychotics — [see Contraindications (4)]
Anticonvulsants —
Lithium —
Drugs Tightly Bound to Plasma Proteins — [see Clinical Pharmacology (12.3)]
Drugs Metabolized by CYP3A4 — in vivo
in vitro
Olanzapine —
When using fluoxetine and olanzapine and in combination, also refer to the Drug Interactions section of the package insert for Symbyax.
8 USE IN SPECIFIC POPULATIONS
When using fluoxetine and olanzapine in combination, also refer to the Use in Specific Populations section of the package insert for Symbyax.
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C — 2222
Treatment of Pregnant Women During the Third Trimester
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
[see Clinical Studies (14.1)]
[see Clinical Studies (14.2) ]
[see Clinical Pharmacology (12.3)]
[see Adverse Reactions (6.1)]
[see Warnings and Precautions (5.6)]
[see Box Warning and Warnings and Precautions (5.1)]
2
2
8.5 Geriatric Use
[see Clinical Studies (14.1)][see Clinical Pharmacology (12.4)][see Warnings and Precautions (5.8)]
8.6 Hepatic Impairment
[see Dosage and Administration (2.7) and Clinical Pharmacology (12.4)].
9 DRUG ABUSE AND DEPENDENCE
9.3 Dependence
10 OVERDOSAGE
10.1 Human Experience
10.2 Animal Experience
[see Overdosage (10.3)]
10.3 Management of Overdose
[see Drug Interactions (7.9)]
Physicians’ Desk Reference (PDR)
11 DESCRIPTION
®p17183
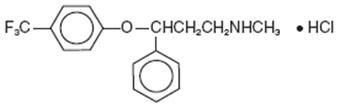
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
1in vitro
12.3 Pharmacokinetics
Systemic Bioavailability
Protein Binding in vitro1
EnantiomersRSS
MetabolismSRSR
Variability in MetabolismSSSR
[see Drug Interactions (7.9)]
Accumulation and Slow Elimination [see Warnings and Precautions (5.12)]
12.4 Specific Populations
Liver Disease [see Dosage and Administration (2.7), Use in Specific Populations (8.6)]
Renal Disease
Geriatric Pharmacokinetics
Pediatric Pharmacokinetics (children and adolescents)
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity 2
Mutagenicity in vivo
Impairment of Fertility 2[see Use in Specific Populations (8.4)].
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
When using fluoxetine and olanzapine in combination, also refer to the Clinical Studies section of the package insert for Symbyax.
14.1 Major Depressive Disorder
Daily Dosing
Adult — .
Pediatric (children and adolescents) —
14.2 Obsessive Compulsive Disorder
Adult
| Fluoxetine | ||||
|---|---|---|---|---|
| Outcome Classification |
Placebo |
20 mg |
40 mg |
60 mg |
| Worse |
8% |
0% |
0% |
0% |
| No change |
64% |
41% |
33% |
29% |
| Minimally improved |
17% |
23% |
28% |
24% |
| Much improved |
8% |
28% |
27% |
28% |
| Very much improved |
3% |
8% |
12% |
19% |
Pediatric (children and adolescents)
14.3 Bulimia Nervosa
14.4 Panic Disorder
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Fluoxetine Capsules USP, 10 mg*
Fluoxetine Capsules USP, 20 mg*
Fluoxetine Capsules USP, 40 mg*
16.2 Storage and Handling
Store at
17 PATIENT COUNSELING INFORMATION
See the FDA-approved Medication Guide.
17.1 General Information
When using fluoxetine and olanzapine in combination, also refer to the Medication Guide for Symbyax.
17.2 Clinical Worsening and Suicide Risk
[see Box Warning and Warnings and Precautions (5.1)].
17.3 Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
[see Warnings and Precautions (5.2) and Drug Interactions (7.3)].
17.4 Allergic Reactions and Rash
[see Warnings and Precautions (5.3)].
17.5 Abnormal Bleeding
[see Warnings and Precautions (5.7) and Drug Interactions (7.6)].
17.6 Hyponatremia
[see Warnings and Precautions (5.8)].
17.7 Potential for Cognitive and Motor Impairment
[see Warnings and Precautions (5.11)].
17.8 Use of Concomitant Medications
17.9 Discontinuation of Treatment
[see Warnings and Precautions (5.13)]
17.10 Use in Specific Populations
Pregnancy [see Use in Specific Populations (8.1) ]
Nursing Mothers [see Use in Specific Populations (8.3)]
Pediatric Use [see Box Warning and Warnings and Precautions (5.1) ][see Warnings and Precautions (5.6) and Use in Specific Populations (8.4)]
® ®
Medication Guide
Fluoxetine Capsules, USP
What is the most important information I should know about fluoxetine capsules?
Antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions:
Talk to your, or your family member’s, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
- How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- or other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes.Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child’s healthcare provider for more information.
What are fluoxetine capsules?
- for short and long-term treatment of depression in adults and children over the age of 8.
- for short and long-term treatment of Obsessive Compulsive Disorder (OCD) in adults and children over the age of 7.
- for short and long-term treatment of Bulimia Nervosa in adults.
- for short-term treatment of Panic Disorder, with or without agoraphobia, in adults.
- with the medicine olanzapine (Zyprexa®), for the short-term treatment of episodes of depression that happen with Bipolar I Disorder.
®
Who should not take fluoxetine capsules?
- Do not take fluoxetine capsules if you take a Monoamine Oxidase Inhibitor (MAOI) or if you stopped taking an MAOI in the last 2 weeks.
- Do not take an MAOI within 5 weeks of stopping fluoxetine capsules. People who take fluoxetine capsules close in time to an MAOI can have serious and life-threatening side effects, with symptoms including:
- high fever
- continued muscle spasms that you can not control
- rigid muscles
- changes in heart rate and blood pressure that happen fast
- confusion
- unconsciousness
- Do not take fluoxetine capsules if you take Mellaril® (thioridazine). Do not take Mellaril within 5 weeks of stopping fluoxetine capsules. Mellaril can cause serious heart rhythm problems and you could die suddenly.
- Do not take fluoxetine capsules if you take the antipsychotic medicine pimozide (Orap®).
What should I tell my doctor before taking fluoxetine capsules?
- seizures (convulsions)
- bipolar disorder (mania)
- are pregnant or plan to become pregnant. It is not known if fluoxetine capsules will harm your unborn baby.
- are breast-feeding or plan to breast-feed. Fluoxetine can pass into your breast milk and may harm your baby. You should not breast-feed while taking fluoxetine capsules. Talk to your doctor about the best way to feed your baby if you take fluoxetine capsules.
Tell your doctor about all the medicines that you take,
If you take fluoxetine capsules, you should not take any other medicines that contain fluoxetine hydrochloride:
- Symbyax®
- Sarafem®
How should I take fluoxetine capsules?
- Take fluoxetine capsules exactly as prescribed. Your doctor may need to change (adjust) the dose of fluoxetine capsules until it is right for you.
- If you miss a dose of fluoxetine capsules, take the missed dose as soon as you remember. If it is almost time for the next dose, skip the missed dose and take your next dose at the regular time. Do not take two doses of fluoxetine capsules at the same time.
- To prevent serious side effects, do not stop taking fluoxetine capsules suddenly. If you need to stop taking fluoxetine capsules, your doctor can tell you how to safely stop taking them.
- If you take too much fluoxetine capsules, call your doctor or poison control center right away, or get emergency treatment.
- Fluoxetine capsules can be taken with or without food.
- Fluoxetine capsules are usually taken once a day, depending on how your doctor prescribes your medicine.
- If you do not think you are getting better or have any concerns about your condition while taking fluoxetine capsules, call your doctor.
What should I avoid while taking fluoxetine capsules?
- Fluoxetine capsules can cause sleepiness and may affect your ability to make decisions, think clearly, or react quickly. You should not drive, operate heavy machinery, or do other dangerous activities until you know how fluoxetine capsules affect you.
What are the possible side effects of fluoxetine capsules?
Fluoxetine capsules may be associated with the following serious risks:
-
Serotonin Syndrome: This is a condition that can be life threatening. Call your doctor right away if you become severely ill and have some or all of these symptoms:
- agitation
- hallucinations
- problems with coordination
- racing heart beat
- over-active reflexes
- fever
- nausea, vomiting, and diarrhea
-
Severe allergic reactions: Tell your doctor right away if you get red itchy welts (hives) or, a rash alone or with fever and joint pain, while taking fluoxetine capsules. Call your doctor right away if you become severely ill and have some or all of these symptoms:
- swelling of your face, eyes, or mouth
- trouble breathing
-
Abnormal bleeding: Tell your doctor if you notice any increased or unusual bruising or bleeding while taking fluoxetine capsules, especially if you take one of these medicines:
- the blood thinner warfarin (Coumadin®, Jantoven®)
- a non-steroidal anti-inflammatory drug (NSAID)
- aspirin
- Mania: You may have a high mood, become extremely irritable, have too much energy, feel pressure to keep talking, or have a decreased need for sleep.
- Seizures
- Loss of appetite
-
Low salt (sodium) levels in the blood (hyponatremia): Call your doctor right away if you become severely ill and have some or all of these symptoms:
- headache
- feel weak
- confusion
- problems concentrating
- memory problems
- feel unsteady
Common possible side effects of fluoxetine capsules include:
How should I store fluoxetine capsules?
- Store fluoxetine capsules at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
- Keep fluoxetine capsules away from light.
- Keep fluoxetine capsules bottle closed tightly.
Keep fluoxetine capsules and all medicines out of the reach of children.
General information about fluoxetine capsules
What are the ingredients in fluoxetine capsules?
Active ingredient:
Inactive ingredients:
Symbyax ® and Sarafem ® are registered trademarks of Eli Lilly and Company.
®
®
®
®
®
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
image of label
FluoxetineFluoxetine Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||